Abstract
Persistent hepatitis B virus (HBV) infection is characterized by a weak and narrowly focused CD8+ T-cell response to HBV that is thought to reflect the induction of central and/or peripheral tolerance to HBV proteins in neonatal and adult onset infections, respectively. Immunotherapeutic strategies that overcome tolerance and boost these suboptimal responses may lead to viral clearance in chronically infected individuals. The present study was performed to compare the relative immunogenicities and tolerogenicities of HBV structural (envelope [ENV]) and nonstructural (polymerase [POL]) proteins at the CD8+ cytotoxic T lymphocyte (CTL) level in transgenic mice that replicate HBV in the liver and secrete infectious virus into the blood, thus representing an excellent model of persistent HBV infection. Interestingly, the mice were tolerant to the ENV but not to the POL proteins at the CTL level. Furthermore, the POL-specific CTLs had no impact on HBV replication or liver function in vivo, even though they were readily induced and reached the liver after DNA immunization, reflecting their relatively low avidity and the low level at which the POL protein is expressed by the hepatocyte. Collectively, these results suggest that the factors that make POL less tolerogenic also make POL-specific CTLs relatively inefficient effector cells when they reach the target organ. Immunotherapeutic strategies to control HBV infection by inducing virus-specific CTL responses in chronically infected subjects should be evaluated in light of this observation.
Cytotoxic T lymphocytes (CTLs) contribute to the control of hepatitis B virus (HBV) infection by killing or inhibiting viral replication in infected cells (14, 25, 27). Acutely infected patients produce a vigorous, polyclonal, and multispecific CTL response to the viral envelope (ENV), nucleocapsid, and polymerase (POL) proteins (25, 27) that is sufficient to clear the infection, while chronically infected patients produce a weak or undetectable CTL response to HBV. Based on these observations, therapeutic induction and/or activation of the T-cell response for one or more of these HBV proteins may have the potential to control HBV infection.
The mechanisms responsible for T-cell hyporesponsiveness or tolerance to HBV proteins in chronic HBV infection are not completely understood, but it is possible that negative selection, immunological ignorance, peripheral anergy, exhaustion, downregulation of cell surface receptors, imbalances in lymphokine production, and defective antigen-presenting cell function can all contribute to hyporesponsiveness in hosts continuously exposed to viral antigens. Knowledge of the extent to which each of these factors contributes to hyporesponsiveness to individual viral proteins should have a profound influence on the design of therapeutic vaccines intended to break immunological tolerance and thereby terminate persistent viral infection.
HBV transgenic mice have previously been produced that express all of the viral proteins and replicate the virus at high levels in their hepatocytes (16). These mice are immunologically tolerant to HBV at the T-cell level and thus represent an excellent model of persistent HBV infection. It has been shown that CD8+ T-cell tolerance to the viral ENV proteins cannot be broken by DNA- or vaccinia virus-based immunization strategies (32, 37). In contrast, tolerance can be broken by immunization with activated dendritic cells (32) or ENV-based lipopeptides (31), but these CTLs are functionally silent. The existence of functionally silent but inducible HBV-specific CTLs in transgenic mice is reminiscent of the emergence of HBV-specific T-cell responses in chronically infected patients during and after treatment with interferon (28) and antiviral drugs (6). Collectively, these observations suggest that peripheral tolerance to the viral ENV proteins might play an important role in the establishment and/or maintenance of chronic HBV infection and that because of their tolerogenic potential, it might be particularly difficult to activate or induce an effective ENV-specific antiviral CTL response in chronically infected patients.
Like other viral POLs (30, 33), the HBV POL protein is a target of CTL response during HBV infection (27). Furthermore, POL appears to be highly immunogenic at the CTL level, since it is produced in trace quantities during HBV infection compared with that of viral structural proteins but induces a comparable CTL response (27). Since POL is required for the earliest steps in the viral life cycle, POL-specific CTL may play an important role in limiting viral spread and thereby attenuating disease severity. The fact that most individuals who are acutely infected by HBV develop a relatively mild, often subclinical, transient infection may be due, at least in part, to the CTL response to this early antigen. Since the CTL response to POL as well as the ENV and nucleocapsid proteins is weak or undetectable in chronically infected patients, strategies designed to enhance the POL-specific CTL response may have therapeutic benefit in chronic HBV infection.
To address this issue, in this study we compared the immunogenicity and tolerogenicity of the HBV ENV and POL proteins in HBV transgenic mice and nontransgenic controls. In the course of these studies, we demonstrated that POL is as immunogenic as ENV in nontransgenic mice after a plasmid DNA prime-vaccinia virus boost immunization. In contrast, under these immunization conditions, ENV was completely nonimmunogenic in the transgenic mice while POL was almost as immunogenic in the transgenic mice as it was in the nontransgenic animals. Nonetheless, the POL-specific CTLs that were induced by immunization did not induce liver disease or inhibit viral replication in the HBV transgenic mice despite the fact that they reached the liver, reflecting their relatively low avidity for the corresponding target cells and the relative low abundance of POL expression by virus-producing cells. These results illustrate that immunogenicity is necessary but not sufficient to induce an effective antiviral CTL response in vivo, suggesting that other variables, including the magnitude of responses, the functional avidity and homing efficiency of the T cells, and the magnitude of epitope display on the target cells, must also be considered.
MATERIALS AND METHODS
Mice.
Nontransgenic BALB/cByJ (H-2d) and C57BL/6 × BALB/cByJ F1 (CB6, H-2bxd) mice and transgenic mice from lineage 1.3.32, which replicate HBV at high levels in the liver without any evidence of cytopathology (16), were used in this study. Lineage 1.3.32 was expanded by repetitive backcrossing against the C57BL/6 parental strain and then bred for one generation against BALB/cByJ mice to produce F1 hybrids (H-2bxd). In all experiments, the mice were matched for age (8 weeks), sex (male), and (in the case of transgenic mice) serum HBV e antigen (HBeAg) levels before experimental manipulation. All animals were housed in pathogen-free rooms under strict barrier conditions.
Plasmids, cell lines, and vaccinia viruses.
Two plasmids that express the entire HBV POL open reading frame (ORF) (amino acids [aa] 1 to 832) were produced. To do this, a fragment containing the entire POL ORF (spanning nucleotides 2290 to 1874 of the Galibert sequence, ayw subtype [12]), was excised by SalI digestion from an EBO-POL construct that was previously described (17). The HBV-POL fragment (POL/ENV) was subcloned into pcDNA3 (Invitrogen, Carlsbad, Calif.) and pCXN2 (kindly provided by Jun-Ichi Miyazaki, Osaka University Medical School [26]), and the resulting recombinant plasmids were named pcDNA3-POL/ENV and pCXN2-POL/ENV, respectively. It is important to note that the POL ORF contains the entire HBV ENV transcription unit and that the POL expression vectors have the capacity to produce both the POL and ENV proteins of HBV (Fig. 1). pcDNA3-POL/ENV was used to immunize mice, and pCXN2-POL/ENV was used to produce stably transfected cell lines (see below). Four additional vectors that contain 3′-end-truncated fragments of POL were produced: pCXN2-POL677 (expressing aa 1 to 677), pCXN2-POL336 (expressing aa 1 to 336), pCXN2-POL189 (expressing aa 1 to 189), and pCXN2-POL109 (expressing aa 1 to 109). A plasmid (pCMV-S2/S) that expresses the middle and major ENV proteins (preS2/S) of HBV under the transcriptional control of the cytomegalovirus immediate early promoter (generously provided by R. Whalen and H. Davis [10, 23] and designated pCMV-ENV herein) was also used to immunize mice (see below).
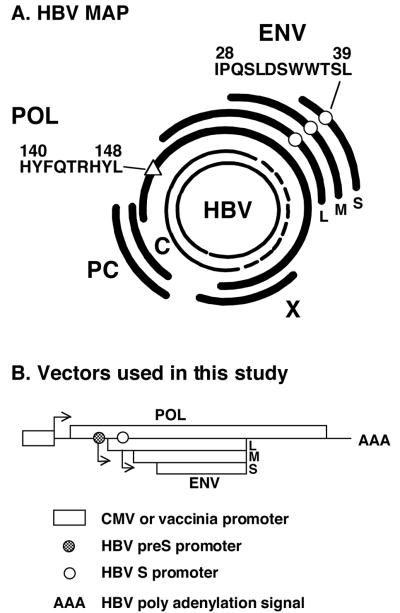
FIG. 1.
(A) HBV map. The partially double-stranded 3.2-kb open circular genome present in circulating virions is shown at the center. The coding regions of viral capsid (C), secreted precore proteins (PC), POL, the large (L), middle (M), and small (S) ENV proteins, and the X protein are shown. The ENV-specific CTL epitope ENV28 (IPQSLDSWWTSL[○]) and POL-specific CTL epitope POL140 (HYFQTRHYL]▵]) are indicated. (B) Map of vectors used in this study. The POL fragment (POL/ENV) was subcloned under the control of the cytomegalovirus promoter or vaccinia virus promoter. The entire HBV ENV transcription unit is contained within the POL transcription unit. Note that the POL ORF contains the entire HBV ENV transcription unit and that the POL expression vectors have the capacity to produce both POL and ENV proteins of HBV.
Using a method similar to that described by Margolskee et al. (21), P815 (H-2d) mastocytoma cells were stably transfected with the panel of full-length and truncated pCXN2-POL vectors. Briefly, 107 P815 cells were washed once and resuspended in 250 μl of electroporation buffer (20 mM HEPES [pH 7.0], 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 6 mM dextrose), 10 μg of each plasmid DNA was added, and cells were electroporated at room temperature in a 0.4-cm-wide cuvette with a Gene Pulser (Bio-Rad, Hercules, Calif.) at 210 V and 960 μF. Stably transfected cell lines were selected with 1 mg of G418 (GIBCO/BRL, Grand Island, N.Y.)/ml. P815 ENV cells that express the large, middle-sized, and major HBV ENV proteins (designated P815ENV herein) and the fibroblast cell lines Dnorm, Knorm, and W12.1, which express the Dd, Kd, and Ld molecules, respectively, were used in this study, and they have been previously described (2, 24).
Wild-type vaccinia virus (WR) and recombinant vaccinia viruses encoding the small ENV protein (vHBs.4 [designated vENV]) and the POL plus HBV ENV protein (vPOL/ENV) of HBV have been described previously (7, 25, 27, 34).
Immunization of nontransgenic and transgenic mice.
All nontransgenic and transgenic mice were immunized once with pCMV-ENV or pcDNA3-POL/ENV. Plasmid DNA (50 μg) was injected into regenerating tibialis anterior muscles (100 μg/mouse) 5 days after the injection of cardiotoxin (10). Two weeks after the first DNA injection, mice were administered a booster injection, either intramuscularly with the same plasmid DNA (100 μg/mouse) or intravenously with 2 × 107 PFU of vaccinia virus encoding the corresponding antigen.
POL-specific and ENV-specific CTLs.
Spleens from POL or ENV-immunized mice were harvested 10 days after the last immunization, and 4 × 106 cells were cocultured with irradiated P815 transfectants (105 cells) that express the corresponding antigen in complete RPMI 1640 medium (GIBCO, Frederick, Md.) containing streptomycin (100 mg/ml), penicillin (100 U/ml), 2-mercaptoethanol (5 × 10−5 M), 10% fetal calf serum, and 2.5% EL-4 supernatant in 24-well plates (Costar, Cambridge, Mass.) and were used as polyclonal effector cells after 5 days of in vitro expansion. Spleen cells were also restimulated with antigen (P815 transfectants) 7 days later, and on day 14 they were cloned in 96-well round bottom plates (Costar) at 1 cell/well. After 2 to 3 weeks of repetitive stimulation, wells containing growing cells were expanded and tested for antigen-specific cytotoxic activity or intracellular gamma interferon (IFN-γ) production as described below. Using this technique, three POL-specific, H-2d-restricted, CD8+ CTL clones were established, and a representative clone (POL2) was used in follow-up experiments. The ENV-specific CD8+ CTL clone (6C2) was used in this study and was previously described (2).
Peptides.
Peptides corresponding to known H-2d-restricted HBV ENV and POL CTL epitope peptides containing predicted Ld and Kd binding motifs were synthesized and purchased from Research Genetics (Huntsville, Ala.).
Lymphomononuclear cell preparation from the liver.
Single cell suspensions were prepared from the spleen and the liver as described previously (19). Briefly, spleen cells were isolated by compressing the spleen against the bottom of a petri dish with the plunger of a 1-ml-diameter syringe. Livers were perfused with 10 ml of phosphate-buffered saline (PBS) via the portal vein to remove circulating lymphocytes and pressed through a 70-μm-pore-diameter Cell Strainer (Becton Dickinson, Franklin Lakes, N.J.). Total liver cells were digested for 40 min at 37°C with 10 ml of RPMI 1640 medium (Life Technologies, Gaithersburg, Md.) containing 0.02% (wt/vol) collagenase IV (Sigma, St Louis, Mo.) and 0.002% (wt/vol) DNase I (Sigma). Cells were washed with RPMI 1640 medium and then underlaid with 24% (wt/vol) metrizamide (Sigma) in PBS. After centrifugation for 20 min at 1,500 × g, intrahepatic lymphocytes (IHLs) were isolated at the interface. Red blood cells were lysed by ACK lysing buffer (0.15 M NH4Cl, 10.0 mM KHCO3, 0.1 mM Na2EDTA [pH 7.2]). The cells were washed once with RPMI 1640 medium and used for further analysis.
Cytotoxicity assays.
Effector cells were cultured with 5 × 103 51Cr-labeled target cells at various effector-to-target cell (E:T) ratios, and the specific cytotoxic activity of the effectors was tested exactly as described previously (24).
Intracellular IFN-γ staining.
IHLs and spleen cells (5 × 105) were cultured in 200 μl of RPMI medium for 5 h in the presence or absence of ENV- and POL-derived peptides (1 μg/ml) or with 5 × 105 P815POL/ENV or P815ENV transfectants or with P815 parental cells in 96-well round-bottom plates (Costar). Fifty units of human recombinant interleukin-2/ml and 1 μl of brefeldin A/ml were added to the cultures. After 5 h, the cells were harvested, washed in PBS (containing 1% BSA and 0.02% sodium azide), and incubated for 20 min on ice with culture supernatant from the hybridoma cell line 2.4G2 (HB-197 [American Type Culture Collection ]) to block nonspecific binding to the Fc receptor. The cells were surface stained with fluorescein isothiocyanate-conjugated monoclonal anti-mouse CD8α antibody (clone 53-6.7; Pharmingen, San Diego, Calif.) for 20 min on ice. After being washed to remove the unbound antibody, the cells were stained with phycoerythrin-conjugated anti-mouse IFN-γ antibody (clone XMG 1.2) and its isotype control antibody (rat IgG1) by using the Cytofix/Cytoperm kit (Pharmingen) according to the manufacturer's instructions. Samples were acquired on a FACScan flow cytometer, and the data were analyzed using CELLQuest software (Becton Dickinson Immunocytometry Systems, San Jose, Calif.).
Injection of ENV- and POL-specific CTLs.
The HBsAg-specific, H-2d-restricted, CD8+ CTL clone 6C2 has been previously described (2). 6C2 and POL2 CTL clones were maintained as previously described (2) by weekly restimulation with irradiated P815 cells that stably express the appropriate antigen. Five days after the last stimulation, the cells were washed, counted, suspended in Hanks balanced salt solution containing 2% fetal calf serum, and injected intravenously into HBV transgenic mice. Three days after injection, mice were sacrificed and their livers were harvested for histological and histochemical analyses or snap frozen in liquid nitrogen and stored at −80°C for subsequent molecular analyses (see below). For experiments in which CTL clones were labeled in vitro with the intracellular dye CFSE (Molecular Probes, Eugene, Oreg.), CFSE (0.5 mM) was added to the cell suspensions and incubated for 10 min at 37°C exactly as described previously (20, 36). The labeling reaction was stopped by repetitive washing with ice-cold RPMI medium-10% fetal calf serum, and cells were washed twice with medium, counted, and injected into the mice. IHLs were harvested 24 h after CTL transfer. Quantitative analysis of the number of CFSE-labeled CTLs was performed by flow cytometry.
Tissue DNA and RNA analyses.
Frozen livers (left lobe) were mechanically pulverized under liquid nitrogen, and total genomic DNA was isolated for Southern blot analysis for HBV DNA exactly as previously described (16). The relative abundance of HBV DNA molecules was quantitated by phosphorimaging analysis, using the Optiquant image analysis software (Packard, Meriden, Conn.).
Biochemical and histological analyses.
The extent of hepatocellular injury was monitored by measuring serum alanine aminotransferase (sALT) activity at multiple time points after treatment. sALT activity was measured in a Paramax chemical analyzer (Baxter Diagnostics Inc., McGaw Park, Ill.) exactly as previously described (15). For histological analysis, liver was fixed in 10% zinc-buffered formalin (Anatech, Battle Creek, Mich.), embedded in paraffin, sliced into 3-μm-thick sections, and stained with hematoxylin and eosin (15).
In vitro MHC-peptide binding assay.
Quantitative assays for the binding of peptides to detergent-solubilized H-2 major histocompatibility complex (MHC) class I molecules (on the basis of the inhibition of a radiolabeled standard probe peptide) were performed as previously described for HLA class I molecules (29). Briefly, 1 to 10 nM radiolabeled probe peptide, iodinated by the chloramine T method, was coincubated for 2 days at room temperature with various amounts of MHC in the presence of 1 μM human β2-microglobulin (Scripps Laboratories, San Diego, Calif.) and a mixture of protease inhibitors. At the end of the incubation period, the percentage of MHC-bound radioactivity was determined by size exclusion gel filtration chromatography on a TSK2000 column (TosoHaas, Montgomeryville, Pa.). The concentration of peptide yielding 50% inhibition (IC50) of the binding of radiolabeled probe in competitive inhibition assays was calculated. Peptides were usually tested at one or two high doses, and the IC50s of peptides yielding positive inhibition were determined in subsequent experiments in which 2 to 6 further dilutions were tested, as necessary. MHC concentrations yielding approximately 15% binding of the radiolabeled probe peptide were used for all competition assays. Each competitor peptide was tested in two to four independent experiments. The radiolabeled probes utilized, and their average IC50s in the respective assays, were as follows: KFNPMKTYI, 1.1 nM for Kd; and FPFKYAAAF, 30 nM for Ld.
RESULTS
Immunization of CB6 mice with POL-encoding vectors generates both POL- and ENV-specific CTLs.
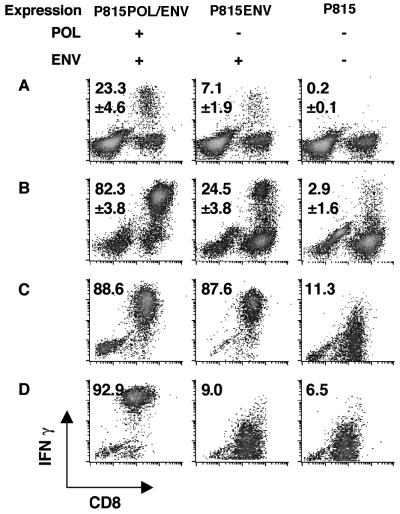
Immunization of mice with a POL-encoding plasmid (pcDNA3-POL/ENV) and recombinant vaccinia virus (vPOL/ENV) has the potential to generate not only POL-specific CTLs but also ENV-specific CTLs, because the entire HBV ENV transcription unit is contained within the POL transcription unit in the HBV genome and, therefore, within the vectors used in this study (Fig. 1). To determine whether the immunization of nontransgenic mice (CB6) with pcDNA3-POL/ENV and vPOL/ENV could generate POL-specific and/or ENV-specific CTLs, three 8-week-old male CB6 mice were immunized with the plasmid and vaccinia virus at 2-week intervals and their spleens were harvested 10 days after the second injection (see Materials and Methods for details). CB6 mice were chosen because they are syngeneic to the HBV transgenic mice (lineage 1.3.32) that were used in follow-up experiments (see below). Spleen cells from these animals were incubated for 5 h with P815 transfectants that express either POL plus ENV (P815POL/ENV) or ENV alone (P815ENV) and analyzed ex vivo for antigen recognition by intracellular IFN-γ staining. As shown in Fig. 2A, this analysis revealed that CD8+ T cells capable of recognizing both P815POL/ENV (23.3% ± 4.6%, mean ± standard deviation [SD]) and, to a lesser extent, P815ENV (7.1% ± 1.9%) were readily detectable in these spleens. After 5 days of in vitro stimulation with P815POL/ENV, 82.3% ± 3.8% of CD8+ spleen cells responded to P815POL/ENV and produced IFN-γ, while 24.5% ± 3.8% of CD8+ cells responded to P815ENV (Fig. 2B). The fact that the percentage of CD8+ cells responding to P815POL/ENV was higher than that of CD8+ cells responding to P815ENV suggests that POL-specific CTLs had been induced in these mice. To specifically detect POL-specific CTLs in this system, however, it was necessary to demonstrate that the CTLs recognize POL-specific epitopes, because ENV-specific CTLs are activated by P815POL/ENV as well as P815ENV transfectants (Fig. 2C) due to the presence of the ENV transcription unit internally in the POL expression vectors. To perform this demonstration, we established POL-specific CTL clones.
FIG. 2.
Induction of POL-specific and ENV-specific CTLs. (A) Three CB6 F1 mice were immunized with pcDNA3-POL/ENV followed by vPOL/ENV, both of which express the HBV POL plus ENV proteins. Ten days after the last immunization, the frequency of CD8+ T cells that responded to POL and ENV protein ex vivo was measured by intracellular IFN-γ staining. Spleen cells from immunized mice were incubated with the indicated transfectants for 5 h and stained for intracellular IFN-γ. Data are presented as the percentage of CD8+ T cells that produce IFN-γ upon stimulation with transfectants that express POL and/or ENV proteins. Representative responses of spleen cells from one mouse are shown. The mean number of antigen-specific CD8+ T cells for the three mice is shown in each panel. (B) The frequency of POL-specific and/or ENV-specific CD8+ T cells after 5 days of in vitro stimulation with P815POL/ENV was measured by intracellular IFN-γ staining. Cultured cells were incubated with indicated transfectants for 5 h and stained for intracellular IFN-γ. Results are shown as described above. (C) ENV28- to 39-specific CTL clone 6C2 can recognize both P815POL/ENV and P815ENV. 6C2 cells (5 × 105) were incubated with same number of indicated transfectants for 5 h and stained for intracellular IFN-γ. Results are representative of at least three independent experiments. (D) The POL-specific CTL clone POL2 responded to P815POL/ENV but not to P815ENV. POL2 (5 × 105) cells were incubated with same number of indicated transfectants for 5 h and stained for intracellular IFN-γ. Results are representative of at least three independent experiments.
Production and characterization of POL-specific CTL clones.
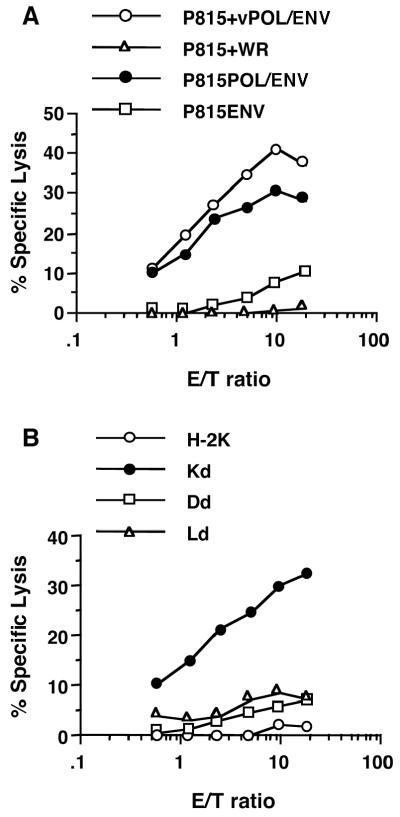
Three POL-specific CTL clones were established as described in Materials and Methods. Since their respective levels of cytolytic activity and IFN-γ production were identical, a representative clone (POL2) was used in subsequent experiments. As shown in Fig. 2D, POL2 recognized P815POL/ENV but not P815ENV or the parental P815 cells. In addition, POL2 killed P815POL/ENV and P815 cells infected by vPOL/ENV but not P815ENV or P815 cells infected by control WR (Fig. 3A). POL2 was restricted by the Kd molecule, as indicated by the specific killing of Kd cells that were infected with vPOL/ENV (Fig. 3B). In contrast, little or no killing was detected when POL2 was incubated with vPOL/ENV-infected Dd or Ld cells (Fig. 3B).
FIG. 3.
Characterization of POL-specific CTL. (A) POL specificity was confirmed by 51Cr release assays against P815 cells infected by vPOL/ENV or WR and against P815POL/ENV and P815ENV cells. (B) H-2 restriction of POL-specific CTLs was determined by monitoring 51Cr release from a panel of fibroblast cell lines. Parental cell KOL (○) expressed only H-2k. Dnorm (□), Knorm (•), and W12.1 (▵) cells expressed the Dd, Kd, and Ld molecules, respectively.
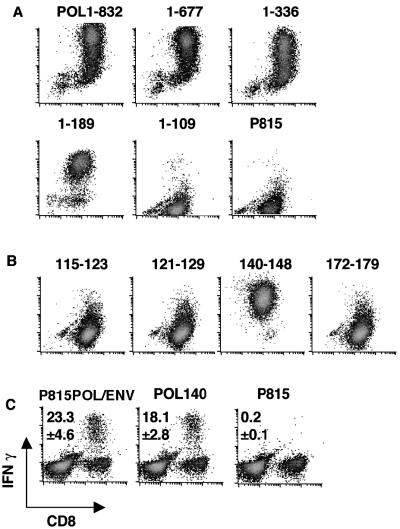
Using P815 cells transfected with 3′-end-truncated fragments of POL (P815POL.1 to 677, P815POL.1 to 336, P815POL.1 to 189, and P815POL.1 to 109; see Materials and Methods) as stimulator cells, we found that the smallest fragment of POL recognized by POL2 was that between aa 1 and 189 (Fig. 4A). Conversely, no antigen recognition was detected when POL2 was incubated with P815POL.1 to 109, which expresses only the first 109 aa of POL (Fig. 4A). These results indicate that the POL-specific CTL epitope is contained within aa 109 to 189 of POL.
FIG. 4.
Fine specificity of POL-specific CTL. (A) P815POL truncation series. The POL2 CTL clone was incubated with the indicated transfectants, which express full-length POL proteins (P815POL1 to 832) and C-terminally truncated fragments of POL (P815POL.1 to 677, P815POL.1 to 336, P815POL.1 to 189, and P815POL.1 to 109), for 5 h and stained for intracellular IFN-γ. (B) Kd motif-containing peptides between POL109 and 189. The POL2 CTL clone was incubated with four Kd binding peptides (POL.115 to 123 [FYPKVTKYL], POL.121 to 129 [KYLPLDKGI], POL.140 to 148 [HYFQTRHYL], and POL.172 to 179 [PYSWEQDL]) for 5 h and stained for intracellular IFN-γ. (C) Three CB6 F1 mice were immunized with pcDNA3-POL/ENV followed by vPOL/ENV. Ten days after the last immunization, the frequencies of CD8+ T cells that responded to P815POL/ENV and POL140 peptide ex vivo were measured by intracellular IFN-γ staining. Spleen cells from immunized mice were incubated with indicated peptide or transfectants for 5 h and stained for intracellular IFN-γ. Representative responses of spleen cells from one mouse are shown. The mean number of CD8+ T cells that produce IFN-γ upon stimulation with peptide or transfectants that express POL and/or ENV proteins is shown in each panel.
The antigenic fine specificity of POL2 was further defined using synthetic peptides that are located between aa 109 and 189 of POL and display the Kd binding motif -Y————(V, I, A, L) (11). By scanning the POL sequence for the presence of Kd motif peptides, four candidate epitope peptides were identified: POL.115 to 123 (FYPKVTKYL), POL.121 to 129 (KYLPLDKGI), POL.140 to 148 (HYFQTRHYL), and POL.172 to 179 (PYSWEQDL). The fine specificity of POL2 was monitored by intracellular IFN-γ staining using P815 cells pulsed with the four peptides. This analysis revealed that peptide POL.140 to 148 represents the CTL epitope recognized by POL2 (Fig. 4B).
It is important to note that most of the H-2d-restricted, POL-specific CTLs detected ex vivo in the spleens of pcDNA3-POL/ENV-immunized CB6 mice recognize peptide POL.140 to 148 (Fig. 4C). Indeed, 18.1% ± 2.8% of CD8+ T cells from POL-immunized ex vivo spleen cells recognized peptide POL140 among the 23.3% ± 4.6% of CD8+ T cells that recognized P815POL/ENV transfectants. These results indicate that this peptide represents a dominant H-2d-restricted, POL-specific CTL epitope and that POL/ENV immunization preferentially induced POL-specific rather than ENV-specific CTLs.
POL and ENV are comparably immunogenic in nontransgenic mice.
Having developed the reagents to induce and monitor POL- and ENV-specific CTLs, we were able to compare the relative immunogenicities of viral structural (ENV) and nonstructural (POL) proteins in the same animals. Four 8-week-old nontransgenic male CB6 mice were injected with either pCMV-ENV or pcDNA3-POL/ENV DNA and given booster injections with the corresponding vaccinia virus. Their spleens were harvested 10 days after the last injection and analyzed ex vivo (see Materials and Methods for details). As shown in Fig. 5A, 25.1% ± 2.9% or 11.6% ± 2.2% of CD8+ spleen cells responded to ENV28 after either ENV immunization or POL immunization, respectively. Importantly, a comparable number (17.8% ± 2.8%) of CD8+ spleen cells recognize POL140 in the POL-immunized mice (Fig. 5A), demonstrating that POL is as immunogenic as ENV in nontransgenic CB6 F1 mice.
FIG. 5.
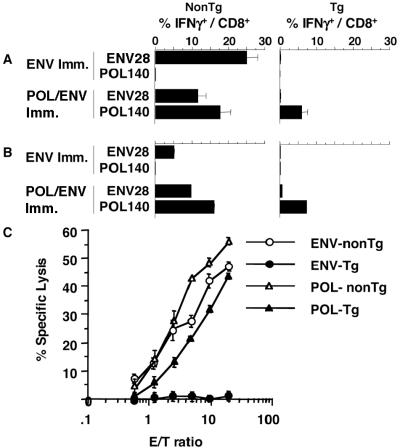
Immunogenicity of POL/ENV immunization in HBV transgenic and nontransgenic mice. (A) Three CB6 F1 nontransgenic mice (left column) or three CB6 F1 HBV transgenic mice (right column) were immunized with plasmid DNA encoding the HBV ENV or POL proteins and administered booster injections with vaccinia virus encoding corresponding proteins. Ten days after the last immunization, the frequency of splenic CD8+ T cells that responded to ENV or POL protein was measured by intracellular IFN-γ staining ex vivo. Spleen cells from immunized mice were incubated with the indicated epitope peptide for 5 h and stained for intracellular IFN-γ. Data are presented as the percentage of CD8+ T cells that produce IFN-γ upon stimulation with each epitope peptide. (B) The frequencies of ENV-specific and POL-specific CD8+ T cells in IHLs were analyzed by intracellular IFN-γ staining after DNA-vaccinia virus immunization. IHLs from three mice were harvested, pooled, and used for the assay. (C) The cytotoxic activity of ENV-DNA- and vaccinia virus-immunized spleen cells from three nontransgenic mice (ENV-nonTg) and three HBV transgenic mice (ENV-Tg) and POL-DNA- and vaccinia virus-immunized spleen cells from three nontransgenic mice (POL-nonTg) and three HBV transgenic mice (POL-Tg) was examined after 2 weeks of in vitro expansion. The cytotoxic activity of those cells (three different cell lines per group) was examined against P815 targets that were incubated with 1 μg of ENV28/ml for ENV-specific CTL or 1 μg of POL140 peptide/ml for POL-specific CTL in a 4-h 51Cr release assay, and levels of antigen-specific cytotoxic activity were determined by subtracting the antigen-nonspecific CTL activity against P815 targets at corresponding E:T ratios. The data show means ± SDs of values for three cell lines.
POL is more immunogenic than ENV in HBV transgenic mice.
In contrast to nontransgenic mice, HBV transgenic mice that were immunized with pCMV-ENV or pcDNA3-POL/ENV failed to respond to ENV epitope peptides, while transgenic mice that were immunized with POL/ENV DNA and vaccinia virus responded to POL140 (Fig. 5A, right column), although at somewhat lower levels (5.9% ± 1.6%) than nontransgenic mice (17.8% ± 2.8%). Thus, although POL/ENV immunization induced both POL- and ENV-specific CD8+ T-cell responses in CB6 nontransgenic mice, POL/ENV immunization induced only a POL-specific CD8+ T-cell response in HBV transgenic mice, which implies that POL is more immunogenic and, therefore, less tolerogenic than the ENV protein in these animals.
These results were confirmed when the cytolytic effector function of the transgenic and nontransgenic spleen cells was examined. ENV- or POL/ENV-immunized spleen cells were stimulated in vitro for 2 weeks with P815ENV or P815POL/ENV, respectively, and used as the effector cells in a 51Cr release assay. As shown in Fig. 5C, ENV-specific CTLs that were induced in nontransgenic mice killed P815 cells that were pulsed with ENV28 peptide while spleen cells from ENV-immunized transgenic mice did not kill target cells at all. In contrast, POL/ENV immunization induced fairly comparable POL-specific CTL activities in nontransgenic and HBV transgenic mice, although the nontransgenic control mice displayed approximately fivefold-higher CTL activity than the transgenic mice (Fig. 5C).
Antigen-specific CD8+ cells accumulate in the liver.
Next, studies were performed to determine if the POL-specific CTL induced in the HBV transgenic mice could home to the liver and perform any antiviral effector functions in the immunized animals. Initially, the ability of HBV-specific CD8+ T cells to home to the liver was assessed by monitoring the frequency level of POL140- and ENV28-specific CD8+ T cells in the intrahepatic infiltrate in immunized mice. IHLs from three mice were pooled and used for the assay. As shown in Fig. 5B, we found that antigen-specific CD8+ cells accumulate in the liver after immunization. Antigen-specific cells in the IHLs from mice that were immunized with either ENV or POL/ENV DNA were quantitated by intracellular IFN-γ staining. After ENV immunization in CB6 mice, 5.2% of the CD8+ T cells in the liver recognized ENV28 (Fig. 5B, left column). After POL/ENV-immunization in CB6 mice, 9.7% of the CD8+ T cells in the liver responded to ENV28 and 16.1% of the CD8+ T cells in the liver produced IFN-γ in response to POL140 (Fig. 5B, left column). In keeping with the profound tolerogenicity of the ENV proteins in the transgenic animals, only trace quantities of ENV-specific CD8+ T cells were detectable in their livers after immunization (Fig. 5B, right column) (Table 1, row 1). In contrast, 7.3% of the CD8+ T cells were POL specific in the livers of the transgenic mice (Fig. 5B, right column) (Table 1, row 3), which compared very favorably with the 16.1% POL-specific CD8+ T cells (Fig. 5B, left column) seen in the nontransgenic animals. These results are consistent with recent reports that activated CD8+ T cells can accumulate in the liver in an antigen-nonspecific manner (18, 22), and they indicate that POL-specific CTLs home to the liver normally in the immunized transgenic mice.
TABLE 1.
Intrahepatic Ag-specific IFN-γ+ cells after immunization
| Mouse group | Immunizing agenta | No. of IHLsb | % of CD8+ IHLsb | No. of CD8+ IHLsb | % of IFN-γ+/ CD8+b | No. of IFN-γ+ cells in the liver | Minimum no. of HBV-specific IHLs needed to exert effector functionc |
|---|---|---|---|---|---|---|---|
| 1 | ENV DNA/Vac | 2.2 × 106 | 48 | 1.1 × 106 | 0.09 | 9.5 × 102 | 3.0 × 104 |
| 2 | ENV DNA/DNA | 1.3 × 106 | 7.8 | 1.0 × 105 | 0.02 | 2.0 × 101 | 3.0 × 104 |
| 3 | POL DNA/Vac | 2.5 × 106 | 46 | 1.2 × 106 | 7.3 | 8.4 × 104 | 4.5 × 105 |
| 4 | POL DNA/DNA | 2.0 × 106 | 8.2 | 1.6 × 105 | 2.7 | 4.4 × 103 | 4.5 × 105 |
HBV transgenic mice were immunized by DNA and administered booster injections of vaccinia virus (Vac) or DNA.
IHLs were harvested 10 days after the second immunization, and data represent the averages of results for three or four mice per group.
Data from results presented in Table 2.
Effector function of intrahepatic POL-specific CD8+ T cells.
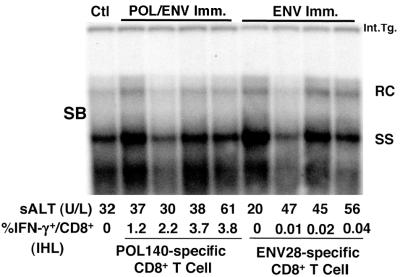
In order to determine whether the POL-specific CD8+ IHLs induced in the HBV transgenic mice can cause hepatitis and control HBV replication, the mice (four mice per group) were analyzed for biochemical and histological evidence of liver cell injury and inflammation and total liver DNA from these animals was analyzed for HBV replication by Southern blotting. Mice were sacrificed 10 days after the second DNA immunization at the peak of intrahepatic antigen-specific CD8+ T-cell response. The number of antigen-specific CD8+ T cells was estimated by multiplying the number of IHLs by the frequencies of CD8+ IHLs and IFN-γ+ cells. As shown in Table 1, row 4, as many as 4.4 × 103 POL-specific cells were estimated to home to the liver. However, sALT activity was not elevated and HBV replication was not inhibited in any of the ENV or POL/ENV DNA-immunized mice, even though POL-specific CD8+ cells were present in the liver (Fig. 6). The POL-specific IHLs were not anergized, since they produced IFN-γ when stimulated with the epitope peptide ex vivo. However, they did not induce hepatitis or inhibit HBV replication in vivo (Fig. 6).
FIG. 6.
Intrahepatic POL-specific CTLs induced in HBV transgenic mice after DNA immunization do not inhibit HBV replication in vivo. CB6 F1 HBV transgenic mice (four mice per group) were immunized twice with either POL/ENV or ENV plasmid DNA. Mice were sacrificed 10 days after the last immunization. Total hepatic DNA was analyzed for HBV DNA by Southern blotting (SB). All DNA samples were RNase treated before quantitation and gel electrophoresis. Bands corresponding to the integrated transgene (Int.Tg.), RC (relaxed-circular) double-stranded HBV DNA, and SS (single-stranded) linear HBV DNA replicative forms are indicated. The integrated transgene can be used to normalize the amount of DNA bound to the membrane. The filter was hybridized with a 32P-labeled HBV-specific DNA probe. Results were compared with those observed in livers pooled from 10 age-, sex-, and serum HBeAg-matched saline-injected transgenic controls (Ctl). The sALT activity values at the time of autopsy are indicated for each mouse and expressed in units/liter (U/L). Intrahepatic Ag-responding cells were also analyzed by intracellular IFN-γ staining. IHLs from immunized animals were incubated for 5 h with epitope peptides (ENV28 for ENV-specific CTLs and POL140 for POL-specific CTL) and stained for intracellular IFN-γ staining. Data are presented as the percentage of CD8+ T cells that produce IFN-γ upon stimulation with each epitope peptide.
MHC binding affinity of ENV and POL epitopes and functional avidity of the corresponding T-cell response.
The foregoing results would be explained if there were major differences in the MHC binding affinities of the ENV and POL peptides or if the avidity of the T cells for the corresponding peptide-MHC complexes were very different. The MHC binding affinities of the ENV28 and POL140 peptides for Ld and Kd were 2.4 nM and 9.1 nM, respectively (not shown). These extremely high binding affinities suggest that both peptides can bind to their corresponding MHC class I molecules very efficiently if they are produced and processed in the cytoplasm.
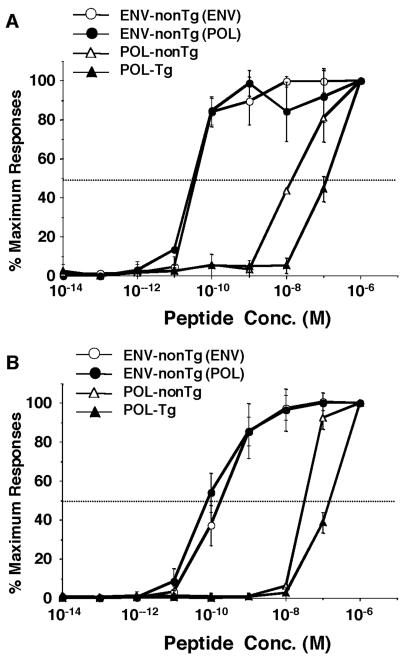
The functional avidities of the ENV- and POL-specific CD8+ T cells were compared in a peptide dose titration experiment in which both cytotoxic activity and the ability to secrete IFN-γ upon stimulation with epitope peptides were monitored (Fig. 7A and B). Spleen cells were harvested 10 days after the second immunization and cultured for 5 days with P815 cells that express the corresponding proteins and were then used as bulk CD8+ CTLs. Nontransgenic ENV-specific CTLs that were induced by ENV immunization [ENV-nonTg (ENV)] or POL/ENV-immunization [ENV-nonTg (POL)] and POL-specific CTLs that were derived from nontransgenic (POL-nonTg) and transgenic mice (POL-Tg) were used as effector cells at an E:T ratio of 5. P815 cells were pulsed with ENV28 peptide or POL140 peptide and used as target cells in a 51Cr release assay with ENV- or POL-specific CTLs, respectively. IFN-γ production was also examined by intracellular IFN-γ staining after incubation of CTLs with the corresponding epitope peptides at the indicated concentration.
FIG. 7.
Functional avidity of ENV and POL-specific CTLs induced in HBV transgenic and nontransgenic animals. (A) The functional avidities of ENV- and POL-specific CD8+ T cells were compared in a peptide dose titration experiment for cytotoxic activity. P815 cells were pulsed with the indicated concentrations of ENV28 peptide for ENV-specific CTLs or POL140 peptide for POL-specific CTLs and used as target cells in a 4-h 51Cr release assay. Nontransgenic ENV-specific CTLs that were induced by ENV immunization [ENV-nonTg (ENV)] or POL immunization [ENV-nonTg (POL)] and POL-specific CTLs that were derived from nontransgenic (POL-nonTg) and transgenic mice (POL-Tg) were used as effector cells at an E:T ratio of 5 with the indicated peptide concentrations. The results (mean ± SD of spleen cells from 3 mice) are expressed as a percentage of the maximum response attained with saturating peptide concentrations (10−6 M). (B) The functional avidities of the ENV- and POL-specific CD8+ T cells were compared in a peptide dose titration experiment, and the ability to secrete IFN-γ upon stimulation with epitope peptides was determined. CTLs were incubated for 5 h with indicated concentrations of epitope peptide and then stained for CD8 and IFN-γ. The data at the indicated peptide concentrations (mean ± SD of spleen cells from three mice) show percentages of maximum IFN-γ production with saturating peptide concentrations (10−6 M).
As shown in Fig. 7A, both ENV-nonTg (ENV) and ENV-nonTg (POL) displayed 50% of maximal specific lysis at 5.0 × 10−11 M. Fifty percent of maximal IFN-γ production of both ENV-nonTg (ENV) and ENV-nonTg (POL) was obtained at 1.0 × 10−10 M. These results suggest that the avidities of ENV-specific CTLs elicited in nontransgenic mice by both the ENV and the POL construct were similar and extremely high. POL-nonTg and POL-Tg CD8+ T cells displayed 50% of maximal specific lysis and 50% of maximal IFN-γ production at 1.0 × 10−8 M and 1.0 × 10−7 M concentrations of the corresponding peptides, respectively (Fig. 7A and B). These results suggest that the functional avidity of ENV-nonTg CTLs is 100 times stronger than that of POL-nonTg CTLs and 1,000 times stronger than that of POL-Tg CTLs, using both cytolytic activity and IFN-γ production as readouts, which perhaps partly explains the failure of the POL-Tg CTLs to express intrahepatic effector function in the POL-immunized transgenic animals.
Minimum number of CTLs necessary to inhibit HBV replication.
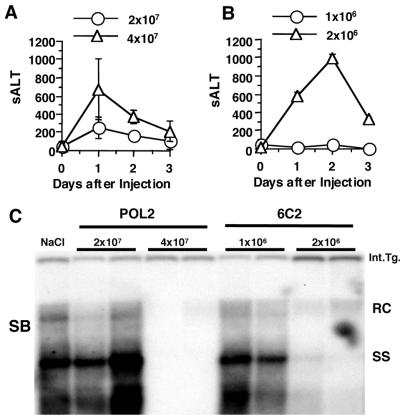
In order to understand why the POL-specific CD8+ IHLs in the immunized HBV transgenic mice failed to cause hepatitis or to inhibit HBV replication in their livers, the minimum number of CTLs necessary to induce liver disease and inhibit HBV replication in the liver of transgenic mice was evaluated. CTL clones that have full effector function were used in this particular experiment instead of bulk CD8+ spleen cells to estimate the minimum number of CTLs able to exert antiviral effector function in HBV transgenic mouse livers. As shown in Fig. 8, adoptive transfer of as few as 2 × 106 ENV-specific CTL clones (clone 6C2) induced a necroinflammatory liver disease and inhibited HBV replication in the HBV transgenic recipients, whereas 4 × 107 POL-specific CTL clones (clone POL2) were required to achieve a comparable effect. In order to determine the minimum number of CTLs that must reach the liver in order to induce liver disease and inhibit HBV replication in the HBV transgenic mice, 2 × 106 ENV-specific and 4 × 107 POL-specific nontransgenic CTLs were labeled with CFSE and transferred intravenously to HBV transgenic mice (3 mice per group). IHLs were harvested from the liver 24 h after CTL transfer and CFSE+ cells were identified by flow cytometry. The number of CFSE+ cells in the IHL population was 3.0 ± 2.0 × 104 after 6C2 transfer and 4.5 ± 1.6 × 105 after POL2 transfer (Table 2). Note that 15-fold more POL-specific CTLs needed to accumulate in the liver to have the same effect as the ENV-specific CTLs (Table 2). Furthermore, the minimal number of POL-specific CD8+ T cells needed to exert an antiviral effect in the liver after adoptive transfer was at least 5-fold higher than the number of POL-specific CD8+ T cells that homed to the liver in the immunized transgenic mice (see above, Table 1), thus explaining the apparent lack of an effect of immunization and CTL induction on viral replication and inflammatory liver disease in these animals.
FIG. 8.
Minimum number of CTLs that is necessary to inhibit HBV replication. POL2 CTL clones (2 × 107 or 4 × 107) (A) or 6C2 CTL clones (1 × 106 or 2 × 106) (B) were adoptively transferred to HBV transgenic mice (three mice per group) to examine their ability to cause hepatitis and to control HBV replication in vivo. sALT activity (mean ± SD) at each time point is expressed in units/liter. (C) Total hepatic DNA from the HBV transgenic recipients of the POL (POL2)- and ENV (6C2)-specific CTL clones was analyzed for HBV DNA by Southern blotting (SB). The results for two representative animals per group are shown. All DNA samples were RNase treated before quantitation and gel electrophoresis. Bands corresponding to the integrated transgene (Int.Tg.), RC strain double-stranded HBV DNA, and SS strain linear HBV DNA replicative forms are indicated. The integrated transgene can be used to normalize the amount of DNA bound to the membrane. The filter was hybridized with a 32P-labeled HBV-specific DNA probe. Results were compared with those observed in livers pooled from 10 age-, sex-, and serum HBeAg-matched saline-injected transgenic controls (NaCl).
TABLE 2.
Recruitment of CFSE-labeled CTLs in the liver
| CTLa | No. of transferred CTLs | No. of IHLsb | % of CFSE+ vs IHLb | No. of CFSE+ cells in the liverb |
|---|---|---|---|---|
| 6C2 | 2 × 106 | 5.0 × 106 | 0.6 | 3.0 × 104 |
| POL2 | 4 × 107 | 7.5 × 106 | 5.9 | 4.5 × 105 |
CTLs were labeled with CFSE before transfer.
IHLs were harvested 24 h after transfer and data represent the averages of results for three mice per group.
DISCUSSION
A hallmark of chronic HBV infection is the marked attenuation and narrowness of the CD4+ and CD8+ T-cell response to the virus in contrast to the vigorous and broadly specific T-cell response of acutely infected patients who ultimately clear the infection (9). Based on these observations, the vigor and diversity of the T-cell response to HBV is thought to be responsible for viral clearance, and the failure of that response probably determines viral persistence. While neonatal tolerance mechanisms are thought to be operative in vertically transmitted chronic HBV infections, the immunological basis for T-cell hyporesponsiveness in adult onset chronic infections is poorly understood. Central deletional mechanisms are not likely to be involved, since HBV-specific T-cell responses can be activated during and after spontaneous and therapeutically induced viral clearance and because relatively vigorous T-cell responses are sometimes observed during flares of disease activity in chronically infected patients (28). These observations suggest that peripheral tolerance mechanisms may be operative that render the HBV-specific T cells that are present in these individuals both quantitatively insufficient and functionally incapable of controlling the infection.
If this is correct, immunization strategies that circumvent or break tolerance in chronically infected patients may have the potential to terminate the infection. To be effective, however, these immunization strategies must induce sufficient numbers of antiviral effector T cells of the appropriate specificity, and the T cells must express the required effector functions and home to the liver in adequate numbers, and the corresponding viral epitopes must be sufficiently displayed at the surface of infected cells. Obviously, the nature of the immunogen(s) employed in this setting will have a major impact on the outcome of such intervention. Previous studies indicate that the structural (ENV and nucleocapsid) and nonstructural (POL) proteins of HBV are all highly immunogenic at the CD8+ T-cell level in acutely infected patients (5, 25, 27), that CD8+ T-cell responses to all of these proteins are also detectable in chronically infected patients (albeit at much lower levels) (4, 27), and that these responses are enhanced after spontaneous and interferon-induced resolution of chronic HBV infection (28). None of those studies, however, address the potential immunogenicity or tolerogenicity hierarchies of these different viral proteins; nor do they elucidate whether the various virus-specific responses are equally effective at recognizing and eliminating virus from infected cells.
The present study was undertaken to address those questions. As our model, we used transgenic mice that replicate the HBV in their hepatocytes at levels comparable to those produced by infected patients, because the virus replicates noncytopathically in the liver of these animals (16) and they are immunologically tolerant to the virus (37), so they closely approximate the healthy chronic HBV carrier state. Furthermore, the mice are genetically and immunologically well defined, so precise and reproducible immunological manipulation of the animals is possible. In addition, they express all of the viral proteins, so it is possible to compare the tolerogenicities and immunogenicities of those proteins in the same animals. Finally, the CD8+ T-cell response to the viral ENV protein in these animals (31, 32) and in syngeneic nontransgenic mice has previously been studied, so many of the immune parameters of that response were already known (31, 32). Specifically, it has been shown that ENV-specific CTLs are present in these mice (i.e., they are not deleted) but they are functionally silent, even when they are activated in vivo by lipopeptide or dendritic cell immunization (31, 32) The basis for the ineffective effector function of the ENV-specific CTLs has not been determined in these mice, and the relative abundance, functional avidity, and effector function of ENV-and POL-specific CTLs in this model are entirely unknown.
Therefore, in the present study, we compared the tolerogenicity and immunogenicity of the ENV and POL proteins. There were several additional reasons for the design of the study. First, the ENV and POL proteins represent structural and nonstructural components of the virus. Second, they are produced in much different quantities by infected cells. Indeed, more than 100 ENV proteins are present in each virion in contrast to a single copy of POL, and hundreds or thousands of subviral ENV particles are produced for each POL-containing virion that is secreted by the hepatocytes (8). Third, the entire ENV transcription unit is contained within the POL transcription unit in the HBV genome and, therefore, it is present in the POL expression vectors we used to immunize the mice. Thus, both proteins are expressed by a single vector, thereby enabling us to compare their relative immunogenicity in the same animal under conditions that approximate a natural infection. Nonetheless, the POL-containing plasmid DNA and vaccinia virus vectors we used in this study probably produce more POL protein per ENV protein than occurs during HBV infection (data not shown), because the POL open reading frame is immediately downstream of the transcriptional start sites in those vectors (Fig. 1) while its translation depends on internal initiation within the pregenomic RNA during HBV infection (13).
We began our studies by characterizing the POL-specific CD8+ T-cell response, since the response to this protein had not been previously defined. As shown in Fig. 2A, both POL- and ENV-specific CD8+ T cells were induced in the spleens of nontransgenic mice which had received primary injections of pcDNA-3POL/ENV and booster injections of vPOL/ENV, a result which was consistent with the presence of the POL and ENV coding regions in those vectors. It appeared that POL-specific T-cell response may have been dominant in that experiment, since 23.3% ± 4.6% of the CD8+ spleen cells responded ex vivo to the P815POL/ENV transfectant that expresses both POL and ENV proteins, while only 7.1% ± 1.9% of the cells responded to the P815ENV transfectant that expresses only the ENV proteins. To prove the existence of a POL-specific response and to characterize that response further, the POL-ENV specific T-cell line illustrated in Fig. 2B was cloned by limiting dilution and several CD8+ T clones were produced which recognized POL but not ENV transfectants at the level of IFN-γ production (Fig. 2D) and cytolytic activity (Fig. 3A). The clones were shown to be H-2Kd restricted (Fig. 3B) and specific for an epitope (HYFQTRHYL) located between residues 140 and 148 of the POL protein (Fig. 4).
Using synthetic peptides corresponding to the POL140 epitope and to a previously defined Ld-restricted ENV epitope (IPQSLDSWWTSL) located between residues 28 and 39 of the ENV proteins (2) (Fig. 1) to monitor the POL- and ENV-specific CD8+ T-cell response, we compared the relative immunogenicity of the two proteins in HBV transgenic mice and syngeneic nontransgenic littermate controls. As shown in Fig. 5, ENV immunization of nontransgenic mice induced a CD8+ T-cell response to ENV28, but not to POL140, in the splenic (Fig. 5A, left column) and IHL (Fig. 5B, left column) populations, while POL/ENV immunization induced a response to both of the peptides, consistent with the expression of both proteins by the POL constructs. Strikingly, only the POL140 response was detectable in the spleens (Fig. 5A, right) and livers (Fig. 5B, right column) of comparably immunized transgenic mice. Importantly, although ENV-specific T cells were completely undetectable, the frequencies of POL140-specific CD8+ T cells in the liver and spleen were quite comparable in the two groups of mice, being only two- or threefold lower in the transgenic than in the nontransgenic animals (compare the left and right columns in Fig. 5A and B). These results demonstrate that the HBV transgenic mice were profoundly tolerant to the ENV but not to the POL proteins at the CD8+ T-cell level.
Because it has previously been shown that adoptively transferred nontransgenic HBV ENV28-specific T cells can cause hepatitis (3) and inhibit HBV replication in the liver in the same lineage of HBV transgenic mice by secreting IFN-γ (15), we asked whether similar effector functions were performed by the POL140-specific CD8+ T cells present in the livers of the immunized animals. A slightly different immunization strategy was required to address this question, because the recombinant vaccinia viruses we used in the DNA prime injection-vaccinia virus booster injection protocol cause a transient vaccinia virus-induced inflammatory liver disease that obscures the potential antiviral effect of the HBV-specific T-cell response. For this reason, transgenic mice were immunized by two intramuscular injections of POL/ENV or ENV DNA (which does not cause hepatitis) and they were sacrificed for analysis 10 days later, at the peak of the CD8+ T-cell response. As shown in Fig. 6, compared to those for control mice, there were no significant increases in sALT activity level or any decrease in intrahepatic HBV replication level in any of these animals, even though up to 3.8% of the intrahepatic CD8+ T cells in the POL/ENV-immunized mice were POL140 specific at that time point. Not surprisingly, there were no changes in levels of sALT activity or HBV replication in the ENV-immunized mice whose livers contained no ENV-specific CD8+ T cells (Fig. 6).
There are several possible explanations for the failure of the intrahepatic POL-specific CD8+ T cells to exert detectable effector functions in the livers of the immunized transgenic mice. First, it is possible that the target antigen is not expressed by the hepatocyte. As shown in Fig. 8, however, this hypothesis is not correct, since the nontransgenic POL2 CTL clone caused hepatitis and inhibited HBV replication after adoptive transfer into naïve HBV transgenic mice. It is noteworthy, however, that 4 × 107 of these POL140-specific T cells were required to elicit the same effects as 2 × 106 ENV28-specific T cells from nontransgenic clone 6C2. While these results demonstrate that the Kd-POL140 complex is present at the hepatocyte membrane, the 20-fold difference in levels of effector function of the POL- and ENV-specific CD8+ T cells in vivo could, of course, reflect the different levels of expression of the ENV and POL proteins by the hepatocyte and by potentially different intracellular compartmentalization and antigen presentation pathways of these two proteins.
Second, the transgenic POL140-specific T cell receptors may have low affinity for their cognate antigen. Indeed, peptide titration experiments revealed that POL-immunized transgenic CD8+ T cells bind the Kd-POL140 complex with 10-fold-lower avidity than similarly immunized nontransgenic T cells and with 1,000-fold-lower avidity than that at which ENV-immunized nontransgenic T cells recognize the Ld-ENV28 complex, both at the level of cytolytic activity (Fig. 7A) and peptide-specific IFN-γ production (Fig. 7B). These observations are compatible with the previous finding that HBV ENV28-specific T cells from transgenic mice that were immunized with an ENV28 lipopeptide display a significantly lower avidity for the Ld-ENV28 complex than nontransgenic T cells (31). Interestingly, even though the level of hepatocellular ENV protein production is very high, in previous studies, low-avidity ENV28-specific transgenic T cells did not display any effector function in the liver of immunized mice (31), a result similar to that determined with the POL-immunized animals described herein. Confirmation of the first hypothesis requires quantitation of ENV and POL peptides associated with the corresponding class I molecules at the surface of the hepatocyte. Confirmation of the second hypothesis will require comparative quantitative analysis of the binding avidities of transgenic and nontransgenic ENV-and POL-specific CTLs for the corresponding ENV- and POL-specific class I tetramers. Such experiments were attempted but failed due to our inability to incorporate the POL140 peptide into the Kd binding groove. These studies and additional efforts to produce Kd-POL140 tetramers are under way, and when available, the results will be reported separately.
Finally, it is possible that there are simply not enough POL140-specific CD8+ T cells in the liver for their effector function to be detectable. The following illustration suggests that this hypothesis may be correct. For example, after two rounds of POL/ENV-specific DNA immunization, approximately 1.6 × 105 CD8+ lymphocytes were isolated from the livers of the POL/ENV-immunized transgenic mice, 2.7% (4.4 × 103) of which were POL specific (Table 1). After a POL/ENV-DNA primary injection and a vPOL/ENV booster injection were administered, 8.4 × 104 cells in the liver were POL specific (Table 1). In contrast, 4.5 × 105 CFSE-labeled POL-specific CTLs can be isolated from the livers of transgenic mice 24 h after the adoptive transfer of 4 × 107 CFSE-labeled nontransgenic POL-specific CTL clones (Table 2), which was the minimum number required to cause an increase in sALT activity and a clearance in HBV replication. Therefore, the number of POL140-specific CD8+ T cells that accumulate or survive in the liver of the POL/ENV-immunized mice might simply be insufficient to have an effect.
Thus, the present results indicate that compared with the more abundant HBV ENV polypeptides, the HBV POL protein is relatively nontolerogenic in HBV transgenic mice that replicate the virus at high levels in their livers. Indeed, using DNA immunization, it is relatively easy to induce POL-specific CD8+ T-cell responses in these animals at levels that are quantitatively similar to those induced in nontransgenic mice. Nonetheless, the T cells do not cause hepatitis or inhibit HBV replication in the immunized mice, despite the fact that they accumulate in their livers and can kill target cells and produce IFN-γ when they encounter their cognate antigens in vitro or when derivative CTL clones are infused in large numbers into naïve HBV transgenic mice. The functional silence of the POL-specific T cells in the immunized mice is probably due to their low numbers in the liver (Table 1), their low functional avidity for antigen (Fig. 7), and the low level of expression of the POL protein by the hepatocyte relative to that of the ENV protein (13). Ironically, the low level at which the POL protein is expressed in the liver may be responsible for its low tolerogenicity and high immunogenicity in this model. Despite the induction of POL-specific CD8+ T cells following immunization and the accumulation of up to 105 of the cells in the livers of the transgenic mice (Table 1), the presence of the cells did not cause hepatitis and had no effect on HBV replication (Fig. 6 to 8) (Table 1). We assume this reflects the requirement for accumulation of >4 × 105 POL-specific nontransgenic (higher avidity) CD8+ T cells in the liver for these effector functions to be detectable (Fig. 7) (Table 2). We do not know whether the lack of effector function of the intrahepatic CD8+ POL-specific T cells induced by immunization in the transgenic mice reflects their relatively low abundance or avidity or the relatively low level of POL protein expression by the hepatocyte. Previous results, which demonstrated that high-avidity ENV-specific T cells are also negatively selected in the HBV transgenic environment (31), suggest that even abundant antigen production does not compensate for, and may even contribute to, the low numbers and the low avidity of the virus-specific T cells that accumulate in the liver after immunization.
Additional mechanisms can also help to explain the phenomena observed. For example, in some systems (1, 35), differences in the types of response seem to be due to the natures of the particular viral proteins. In fact, the intracellular localization of the POL protein could be an important factor in the case of the responses described in this study. Differences in the extent of cross-presentation between the different proteins examined could also be relevant. Although the present study did not completely determine the basis of the ineffective effector function of HBV-specific CTLs, it seems that the most economical and likely explanation of the phenomena observed is that such ineffective function stems from a combination of mechanisms related to antigen expression, the magnitude of intrahepatic CD8+ T-cell infiltration, and T-cell receptor avidity.
In conclusion, the present results suggest that therapeutic induction of a CD8+ T-cell response to HBV will not terminate chronic HBV infection unless the T cells accumulate efficiently in the liver and display relatively high functional avidity for their antigens, which must be expressed at sufficiently high levels in order to activate CD8+ T-cell effector mechanisms capable of killing or curing the virus-infected cells. In this light, perhaps therapeutic strategies should be designed to simultaneously target multiple viral antigens. Their aim should be to elicit most vigorous, multispecific response possible, mimicking the multiple specificity and quality of response associated with naturally occurring spontaneous resolution of chronic HBV infection in humans.
Acknowledgments
We thank Stefan Wieland and Luca G. Guidotti for guidance and advice, Jun-ichi Miyazaki for his generous gift in supplying us with pCXN2 vector, Alana Althage, Amber Morris, Masatoshi Ishigami, and Margie Chadwell for excellent technical assistance, and Andrea Achenbach for help with manuscript preparation.
This work was supported by grant CA40489 from the National Institutes of Health.
Footnotes
This is manuscript number 14261-MEM from the Scripps Research Institute.
REFERENCES
- 1.Alwan, W. H., F. M. Record, and P. J. Openshaw. 1993. Phenotypic and functional characterization of T cell lines specific for individual respiratory syncytial virus proteins. J. Immunol. 150:5211-5218. [PubMed] [Google Scholar]
- 2.Ando, K., L. G. Guidotti, S. Wirth, T. Ishikawa, G. Missale, T. Moriyama, R. D. Schreiber, H. J. Schlicht, S. N. Huang, and F. V. Chisari. 1994. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J. Immunol. 152:3245-3253. [PubMed] [Google Scholar]
- 3.Ando, K., T. Moriyama, L. G. Guidotti, S. Wirth, R. D. Schreiber, H. J. Schlicht, S. N. Huang, and F. V. Chisari. 1993. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J. Exp. Med. 178:1541-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertoletti, A., F. V. Chisari, A. Penna, S. Guilhot, L. Galati, G. Missale, P. Fowler, H. J. Schlicht, A. Vitiello, and R. C. Chesnut. 1993. Definition of a minimal optimal cytotoxic T-cell epitope within the hepatitis B virus nucleocapsid protein. J. Virol. 67:2376-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertoletti, A., C. Ferrari, F. Fiaccadori, A. Penna, R. Margolskee, H. J. Schlicht, P. Fowler, S. Guilhot, and F. V. Chisari. 1991. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc. Natl. Acad. Sci. USA 88:10445-10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boni, C., A. Bertoletti, A. Penna, A. Cavalli, M. Pilli, S. Urbani, P. Scognamiglio, R. Boehme, R. Panebianco, F. Fiaccadori, and C. Ferrari. 1998. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J. Clin. Investig. 102:968-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakrabarti, S., K. Brechling, and B. Moss. 1985. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol. Cell. Biol. 5:3403-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chisari, F. V. 2000. Viruses, immunity, and cancer: lessons from hepatitis B. Am. J. Pathol. 156:1117-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chisari, F. V., and C. Ferrari. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13:29-60. [DOI] [PubMed] [Google Scholar]
- 10.Davis, H. L., R. Schirmbeck, J. Reimann, and R. G. Whalen. 1995. DNA-mediated immunization in mice induces a potent MHC class I-restricted cytotoxic T lymphocyte response to the hepatitis B envelope protein. Hum. Gene Ther. 6:1447-1456. [DOI] [PubMed] [Google Scholar]
- 11.Falk, K., O. Rotzschke, S. Stevanovic, G. Jung, and H. G. Rammensee. 1991. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 351:290-296. [DOI] [PubMed] [Google Scholar]
- 12.Galibert, F., E. Mandart, F. Fitoussi, P. Tiollais, and P. Charnay. 1979. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature 281:646-650. [DOI] [PubMed] [Google Scholar]
- 13.Ganem, D., and H. E. Varmus. 1987. The molecular biology of the hepatitis B viruses. Annu. Rev. Biochem. 56:651-693. [DOI] [PubMed] [Google Scholar]
- 14.Guidotti, L. G., and F. V. Chisari. 1996. To kill or to cure: options in host defense against viral infection. Curr. Opin. Immunol. 8:478-483. [DOI] [PubMed] [Google Scholar]
- 15.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4:25-36. [DOI] [PubMed] [Google Scholar]
- 16.Guidotti, L. G., B. Matzke, H. Schaller, and F. V. Chisari. 1995. High-level hepatitis B virus replication in transgenic mice. J. Virol. 69:6158-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guilhot, S., P. Fowler, G. Portillo, R. F. Margolskee, C. Ferrari, A. Bertoletti, and F. V. Chisari. 1992. Hepatitis B virus (HBV)-specific cytotoxic T-cell response in humans: production of target cells by stable expression of HBV-encoded proteins in immortalized human B-cell lines. J. Virol. 66:2670-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, L., G. Soldevila, M. Leeker, R. Flavell, and I. N. Crispe. 1994. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity 1:741-749. [DOI] [PubMed] [Google Scholar]
- 19.Kakimi, K., L. G. Guidotti, Y. Koezuka, and F. V. Chisari. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192:921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171:131-137. [DOI] [PubMed] [Google Scholar]
- 21.Margolskee, R. F., P. Kavathas, and P. Berg. 1988. Epstein-Barr virus shuttle vector for stable episomal replication of cDNA expression libraries in human cells. Mol. Cell. Biol. 8:2837-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehal, W. Z., A. E. Juedes, and I. N. Crispe. 1999. Selective retention of activated CD8+ T cells by the normal liver. J. Immunol. 163:3202-3210. [PubMed] [Google Scholar]
- 23.Michel, M. L., H. L. Davis, M. Schleef, M. Mancini, P. Tiollais, and R. G. Whalen. 1995. DNA-mediated immunization to the hepatitis B surface antigen in mice: aspects of the humoral response mimic hepatitis B viral infection in humans. Proc. Natl. Acad. Sci. USA 92:5307-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriyama, T., S. Guilhot, K. Klopchin, B. Moss, C. A. Pinkert, R. D. Palmiter, R. L. Brinster, O. Kanagawa, and F. V. Chisari. 1990. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science 248:361-364. [DOI] [PubMed] [Google Scholar]
- 25.Nayersina, R., P. Fowler, S. Guilhot, G. Missale, A. Cerny, H. J. Schlicht, A. Vitiello, R. Chesnut, J. L. Person, and A. G. Redeker. 1993. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J. Immunol. 150:4659-4671. [PubMed] [Google Scholar]
- 26.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 27.Rehermann, B., P. Fowler, J. Sidney, J. Person, A. Redeker, M. Brown, B. Moss, A. Sette, and F. V. Chisari. 1995. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J. Exp. Med. 181:1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehermann, B., D. Lau, J. H. Hoofnagle, and F. V. Chisari. 1996. Cytotoxic T lymphocyte responsiveness after resolution of chronic hepatitis B virus infection. J. Clin. Investig. 97:1655-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruppert, J., J. Sidney, E. Celis, R. T. Kubo, H. M. Grey, and A. Sette. 1993. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell 74:929-937. [DOI] [PubMed] [Google Scholar]
- 30.Safrit, J. T., C. A. Andrews, T. Zhu, D. D. Ho, and R. A. Koup. 1994. Characterization of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte clones isolated during acute seroconversion: recognition of autologous virus sequences within a conserved immunodominant epitope. J. Exp. Med. 179:463-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sette, A. D., C. Oseroff, J. Sidney, J. Alexander, R. W. Chesnut, K. Kakimi, L. G. Guidotti, and F. V. Chisari. 2001. Overcoming T cell tolerance to the hepatitis B virus surface antigen in hepatitis B virus-transgenic mice. J. Immunol. 166:1389-1397. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu, Y., L. G. Guidotti, P. Fowler, and F. V. Chisari. 1998. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J. Immunol. 161:4520-4529. [PubMed] [Google Scholar]
- 33.Shirai, M., T. Akatsuka, C. D. Pendleton, R. Houghten, C. Wychowski, K. Mihalik, S. Feinstone, and J. A. Berzofsky. 1992. Induction of cytotoxic T cells to a cross-reactive epitope in the hepatitis C virus nonstructural RNA polymerase-like protein. J. Virol. 66:4098-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, G. L., M. Mackett, and B. Moss. 1983. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature 302:490-495. [DOI] [PubMed] [Google Scholar]
- 35.Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J. Virol. 71:678-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weston, S. A., and C. R. Parish. 1990. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J. Immunol. Methods 133:87-97. [DOI] [PubMed] [Google Scholar]
- 37.Wirth, S., L. G. Guidotti, K. Ando, H. J. Schlicht, and F. V. Chisari. 1995. Breaking tolerance leads to autoantibody production but not autoimmune liver disease in hepatitis B virus envelope transgenic mice. J. Immunol. 154:2504-2515. [PubMed] [Google Scholar]