Abstract
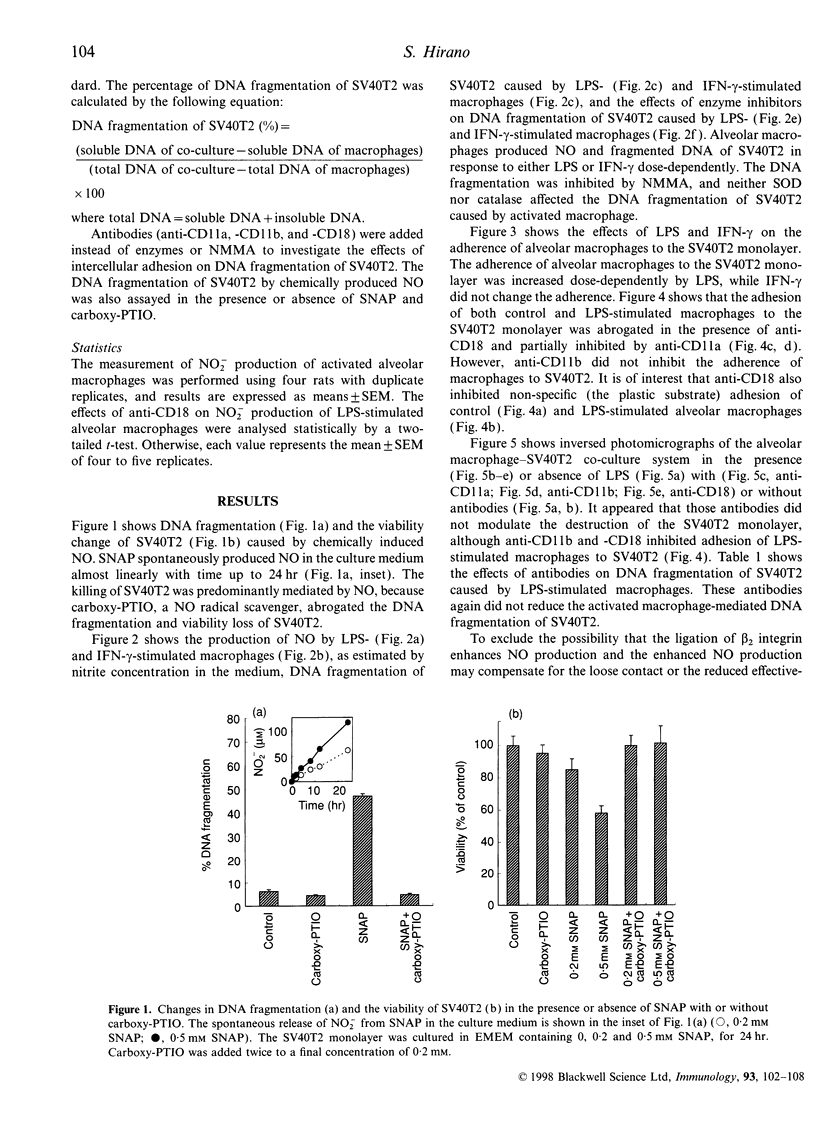
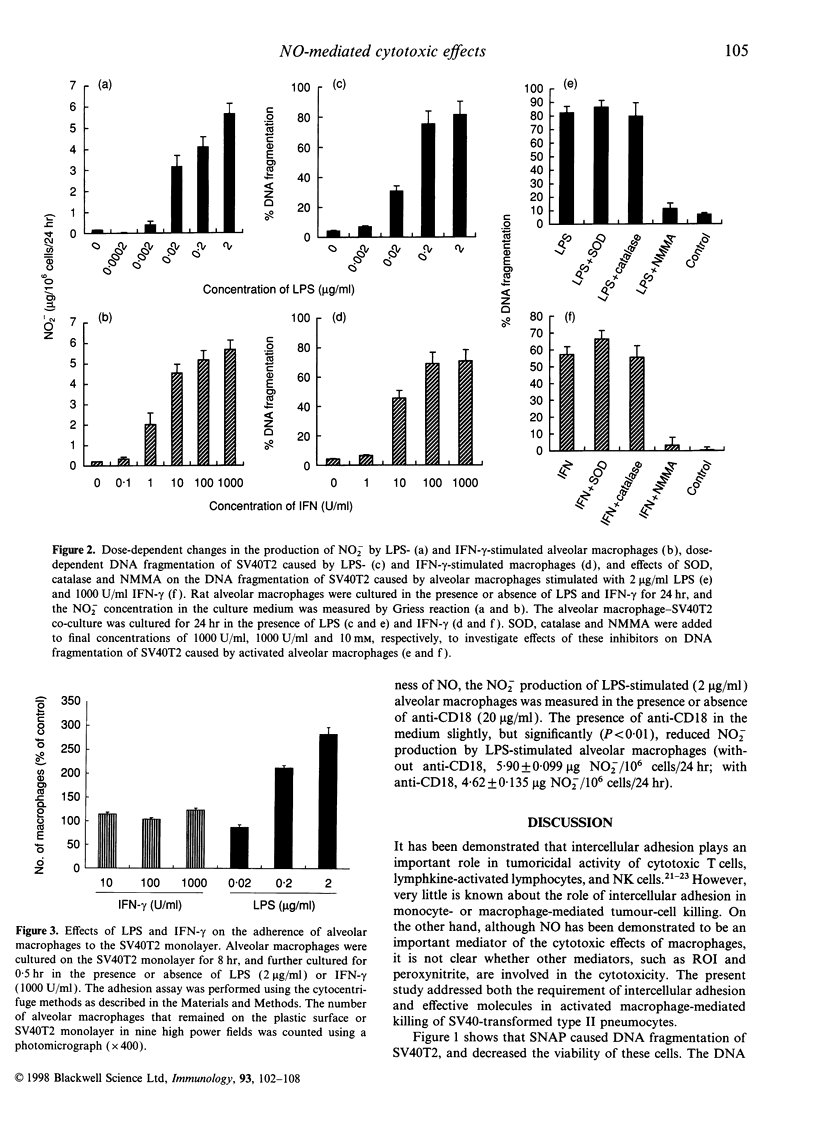
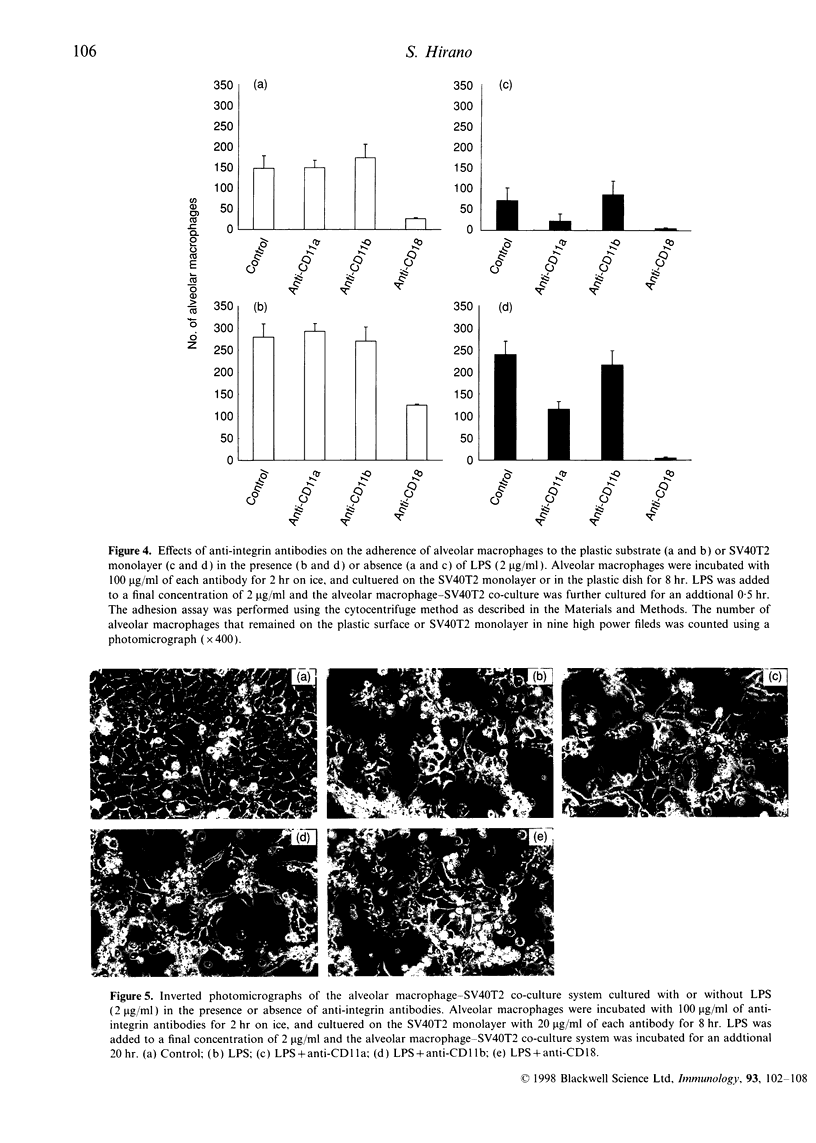
It is known that murine macrophages produce nitric oxide (NO) when stimulated with lipopolysaccharide (LPS) or interferon-gamma (IFN-gamma), and NO mediates the tumoricidal activity of activated macrophages. The present study was designed to investigate whether the intercellular adhesion is necessary for activated rat alveolar macrophages to exert the full cytotoxic effects. Rat alveolar macrophages produced NO dose dependently in response to either LPS or IFN-gamma, and caused DNA fragmentation in rat type II pneumocytes transformed with SV40 (SV40T2). Chemically produced NO also caused the DNA fragmentation and viability loss in SV40T2, and both of them were inhibited by a NO radical scavenger. The cytotoxicity of activated macrophages was reduced by NG-monomethyl-L-arginine, a competitive nitric synthase inhibitor, and neither superoxide dismutase nor catalase modulated the cytotoxicity. Although alveolar macrophages stimulated with either LPS or IFN-gamma caused DNA fragmentation of SV40T2, only LPS increased the intercellular adherence between macrophages and SV40T2. The intercellular adhesion was reduced by both anti-CD18 and anti-CD11a. However, those antibodies did not affect the cytotoxicity of LPS-stimulated macrophages. These results clearly indicate that NO-mediated cytotoxicity is caused predominantly by diffusion of NO, and the beta 2 integrin-mediated intercellular adhesion does not play an important role, if any, in activated macrophage-mediated cytotoxic effects on SV40T2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar-Cohen F. F., Heydolph S., Faure V., Droy-Lefaix M. T., Courtois Y., Goureau O. Peroxynitrite cytotoxicity on bovine retinal pigmented epithelial cells in culture. Biochem Biophys Res Commun. 1996 Sep 24;226(3):842–849. doi: 10.1006/bbrc.1996.1438. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Brown D. M., Dransfield I., Wetherill G. Z., Donaldson K. LFA-1 and ICAM-1 in homotypic aggregation of rat alveolar macrophages: organic dust-mediated aggregation by a non-protein kinase C-dependent pathway. Am J Respir Cell Mol Biol. 1993 Aug;9(2):205–212. doi: 10.1165/ajrcmb/9.2.205. [DOI] [PubMed] [Google Scholar]
- Clement A., Steele M. P., Brody J. S., Riedel N. SV40T-immortalized lung alveolar epithelial cells display post-transcriptional regulation of proliferation-related genes. Exp Cell Res. 1991 Oct;196(2):198–205. doi: 10.1016/0014-4827(91)90251-o. [DOI] [PubMed] [Google Scholar]
- Dinapoli M. R., Calderon C. L., Lopez D. M. The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. J Exp Med. 1996 Apr 1;183(4):1323–1329. doi: 10.1084/jem.183.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C. Cell-mediated cytotoxicity. Cell. 1993 Nov 19;75(4):607–612. doi: 10.1016/0092-8674(93)90480-e. [DOI] [PubMed] [Google Scholar]
- Edelman J., Cardozo C., Lesser M. Lipopolysaccharide stimulates alveolar macrophage adherence in vivo and in vitro. Agents Actions. 1989 Mar;26(3-4):287–291. doi: 10.1007/BF01967292. [DOI] [PubMed] [Google Scholar]
- Farias-Eisner R., Chaudhuri G., Aeberhard E., Fukuto J. M. The chemistry and tumoricidal activity of nitric oxide/hydrogen peroxide and the implications to cell resistance/susceptibility. J Biol Chem. 1996 Mar 15;271(11):6144–6151. doi: 10.1074/jbc.271.11.6144. [DOI] [PubMed] [Google Scholar]
- Gaston B., Drazen J. M., Loscalzo J., Stamler J. S. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med. 1994 Feb;149(2 Pt 1):538–551. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Hirano S. Interaction of rat alveolar macrophages with pulmonary epithelial cells following exposure to lipopolysaccharide. Arch Toxicol. 1996;70(3-4):230–236. doi: 10.1007/s002040050265. [DOI] [PubMed] [Google Scholar]
- Ioannidis I., de Groot H. Cytotoxicity of nitric oxide in Fu5 rat hepatoma cells: evidence for co-operative action with hydrogen peroxide. Biochem J. 1993 Dec 1;296(Pt 2):341–345. doi: 10.1042/bj2960341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Geiges M., Keist R. L-arginine-dependent reactive nitrogen intermediates as mediators of tumor cell killing by activated macrophages. Cancer Res. 1990 Mar 1;50(5):1421–1425. [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- Lowenstein C. J., Snyder S. H. Nitric oxide, a novel biologic messenger. Cell. 1992 Sep 4;70(5):705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- Piessens W. F. Increased binding and killing of neuraminidase-galactose oxidase-treated tumor cells by normal macrophages. J Immunol. 1977 Jul;119(1):167–172. [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991 Mar 5;266(7):4244–4250. [PubMed] [Google Scholar]
- Rosen H., Gordon S. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J Exp Med. 1987 Dec 1;166(6):1685–1701. doi: 10.1084/jem.166.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Walter U. NO at work. Cell. 1994 Sep 23;78(6):919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Sconocchia G., Titus J. A., Segal D. M. CD44 is a cytotoxic triggering molecule in human peripheral blood NK cells. J Immunol. 1994 Dec 15;153(12):5473–5481. [PubMed] [Google Scholar]
- Shaw L. M., Messier J. M., Mercurio A. M. The activation dependent adhesion of macrophages to laminin involves cytoskeletal anchoring and phosphorylation of the alpha 6 beta 1 integrin. J Cell Biol. 1990 Jun;110(6):2167–2174. doi: 10.1083/jcb.110.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille Y., Reynolds H. Y. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990 Feb;141(2):471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summersgill J. T., Powell L. A., Buster B. L., Miller R. D., Ramirez J. A. Killing of Legionella pneumophila by nitric oxide in gamma-interferon-activated macrophages. J Leukoc Biol. 1992 Dec;52(6):625–629. doi: 10.1002/jlb.52.6.625. [DOI] [PubMed] [Google Scholar]
- Szabó C., Salzman A. L. Endogenous peroxynitrite is involved in the inhibition of mitochondrial respiration in immuno-stimulated J774.2 macrophages. Biochem Biophys Res Commun. 1995 Apr 17;209(2):739–743. doi: 10.1006/bbrc.1995.1561. [DOI] [PubMed] [Google Scholar]
- Yu C. P., Chen Y. K., Morrow P. E. An analysis of alveolar macrophage mobility kinetics at dust overloading of the lungs. Fundam Appl Toxicol. 1989 Oct;13(3):452–459. doi: 10.1016/0272-0590(89)90282-0. [DOI] [PubMed] [Google Scholar]
- Zarcone D., Viale O., Cerruti G., Tenca C., Malorni W., Arancia G., Iosi F., Galandrini R., Velardi A., Moretta A. Antibodies to adhesion molecules inhibit the lytic function of MHC-unrestricted cytotoxic cells by preventing their activation. Cell Immunol. 1992 Sep;143(2):389–404. doi: 10.1016/0008-8749(92)90035-n. [DOI] [PubMed] [Google Scholar]



