Abstract
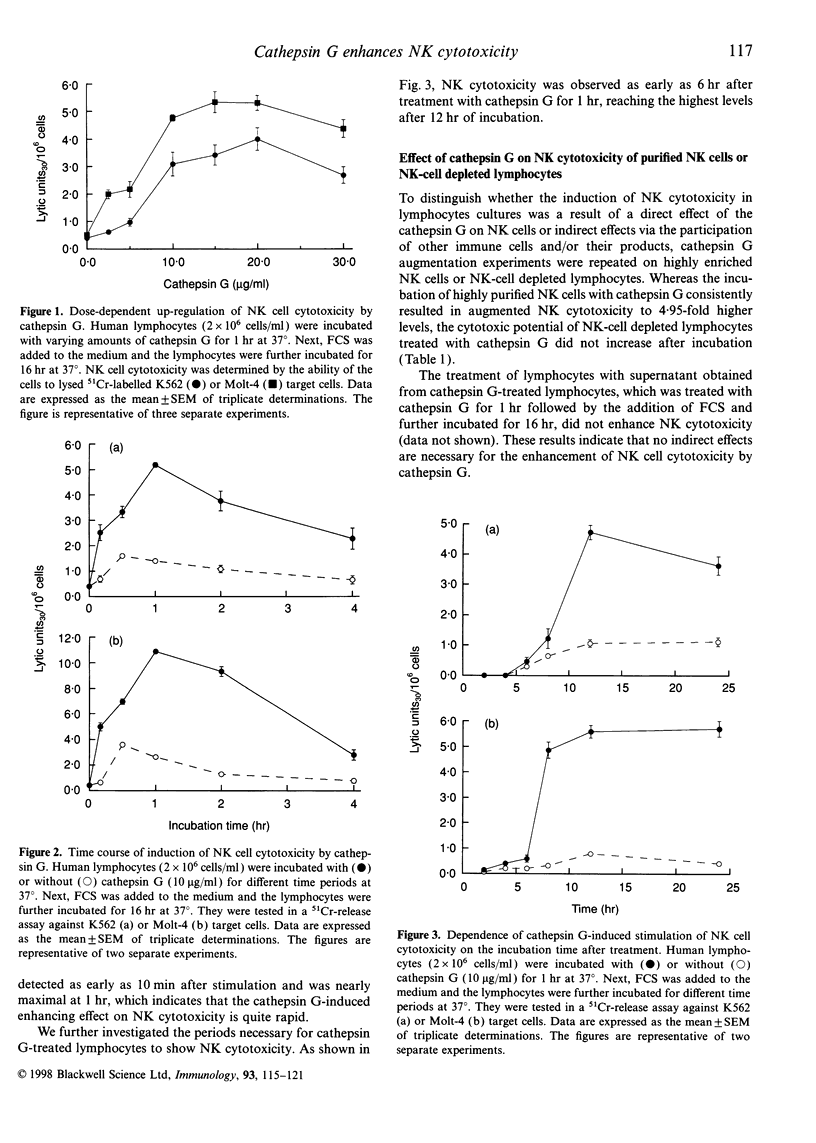
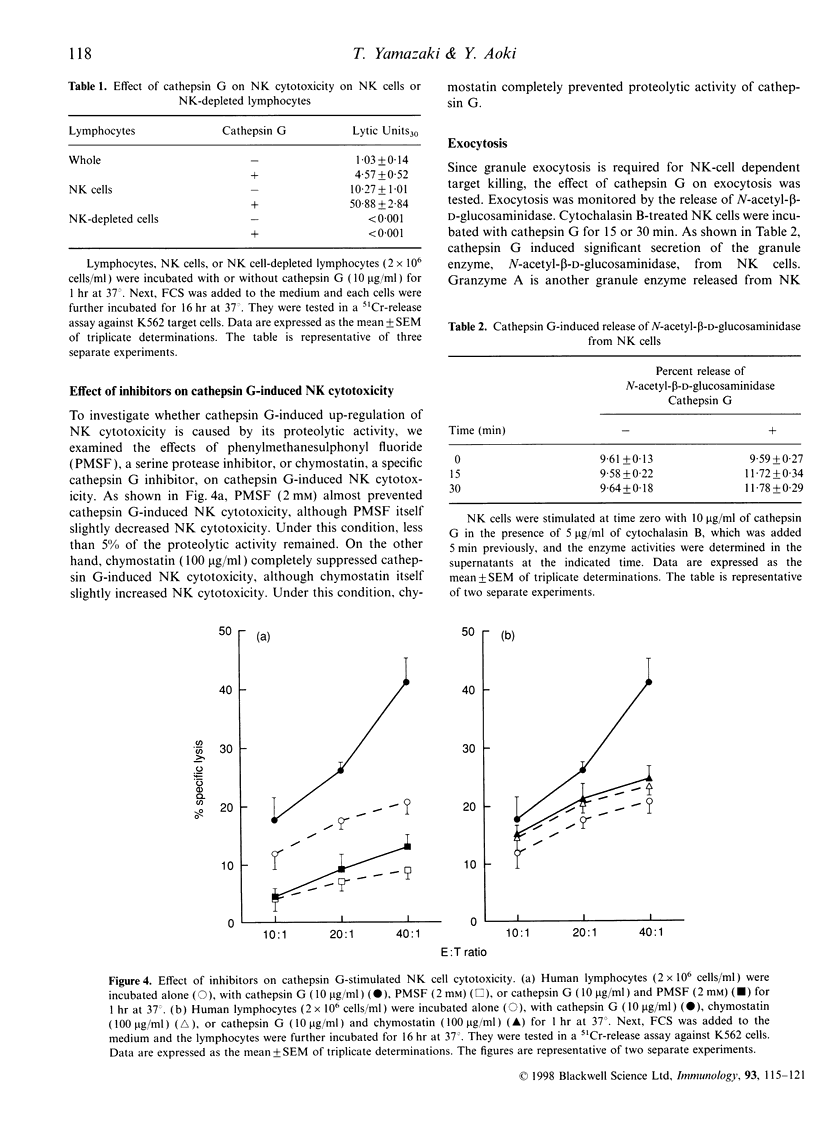
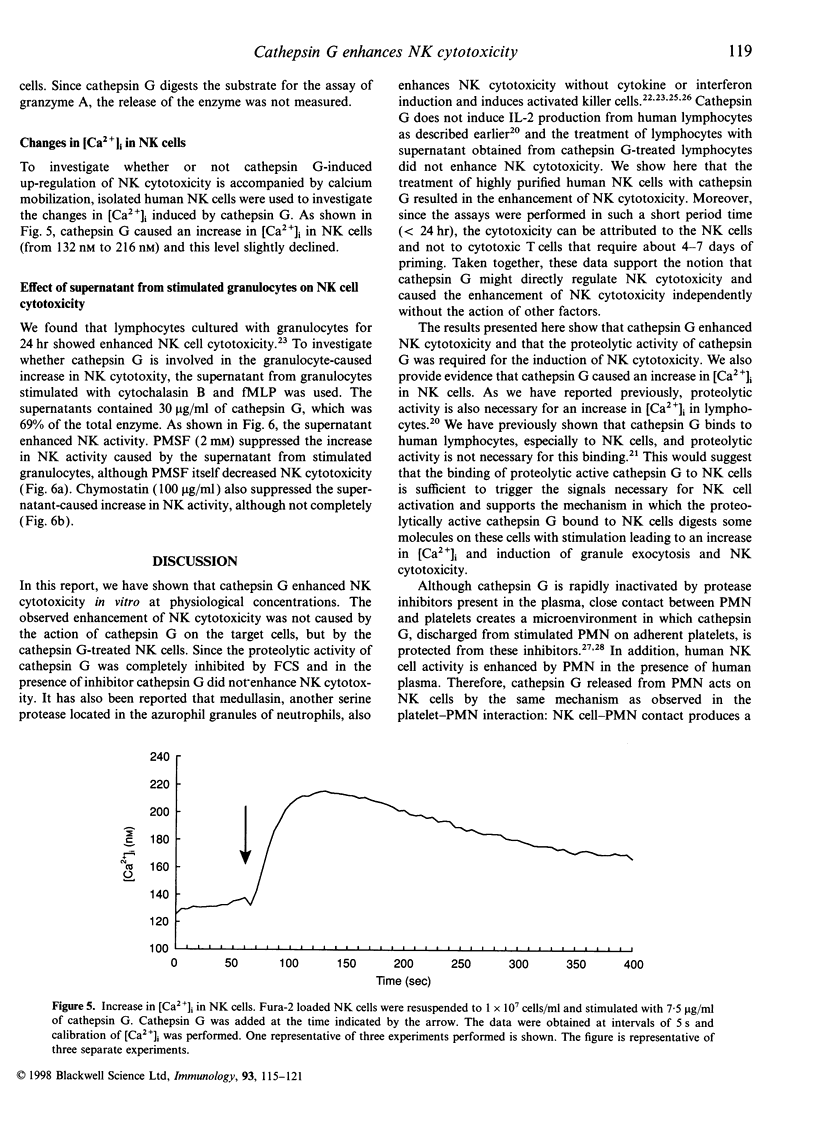
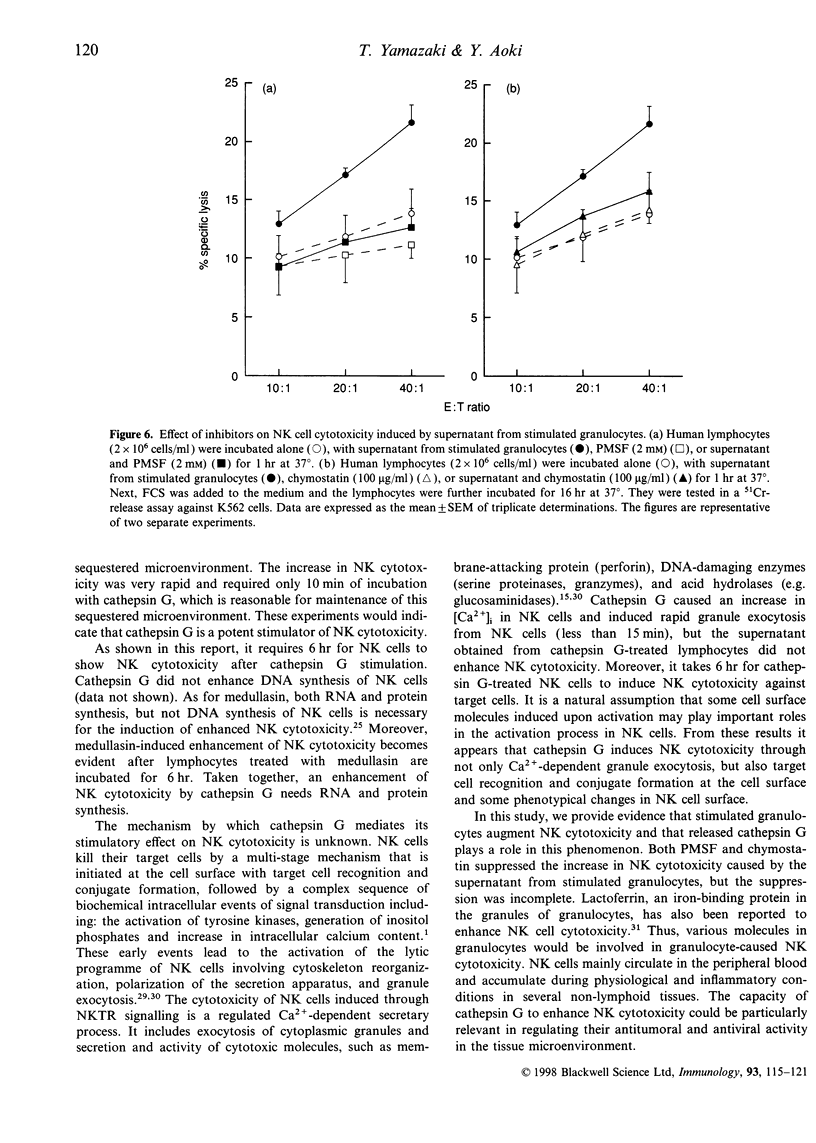
Cathepsin G is a serine protease located in the azurophil granules of neutrophils. In this study, we investigated the effect of cathepsin G on the functions of human natural killer (NK) cells in vitro. Cathepsin G enhanced NK cytotoxicity rapidly in a dose-dependent fashion. The ability to augment NK cytotoxicity was markedly reduced in the presence of the inhibitor, phenylmethanesulphonyl fluoride (PMSF) or chymostatin, demonstrating that the proteolytic activity of cathepsin G is essential for the induction of NK cytotoxicity. Granulocyte exocytosis is required for NK cell-dependent target killing. Cathepsin G induced the release of the granule enzyme, N-acetyl-beta-D-glucosaminidase, from human NK cells. Moreover, an increase in the cytosolic-free Ca2+ concentration was observed in NK cells after stimulation with cathepsin G. When human granulocytes were stimulated with cytochalasin B and N-formyl-methionyl-leucyl-phenylalanine (fMLP), cathepsin G was released. The cathepsin G released from granulocytes also caused enhancement of NK cytotoxicity. In the presence of serine protease inhibitor the supernatant including cathepsin G obtained from stimulated granulocytes did not enhance NK cytotoxicity, but the stimulated granulocytes did. Highly purified human NK cells treated with cathepsin G enhanced NK cytotoxicity, but NK-depleted lymphocytes did not, demonstrating that cathepsin G regulates NK cytotoxicity independently of other factors. We have shown recently that human cathepsin G binds to human NK cells. These combined data indicate that cathepsin G released from granulocytes binds to NK cells and augments NK cytotoxicity through its protease activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki Y., Fukuchi K. Medullasin enhances human natural killer cell activity by a mechanism other than the induction of interferons or interleukin-2. Jpn J Cancer Res. 1987 Jan;78(1):68–73. [PubMed] [Google Scholar]
- Aoki Y., Hase T., Oshimi K., Suzuki K. Induction of activated killer cells from human lymphocytes by medullasin (a serine protease in bone marrow cells). Immunology. 1992 Mar;75(3):481–487. [PMC free article] [PubMed] [Google Scholar]
- Aoki Y., Sumiya M., Oshimi K. Medullasin enhances human natural killer cell activity. J Clin Invest. 1982 Jun;69(6):1223–1230. doi: 10.1172/JCI110561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y. The mechanism of activation of human natural killer cells by medullasin. Jpn J Cancer Res. 1986 Oct;77(10):1018–1026. [PubMed] [Google Scholar]
- Berke G. The CTL's kiss of death. Cell. 1995 Apr 7;81(1):9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- Bonnema J. D., Rivlin K. A., Ting A. T., Schoon R. A., Abraham R. T., Leibson P. J. Cytokine-enhanced NK cell-mediated cytotoxicity. Positive modulatory effects of IL-2 and IL-12 on stimulus-dependent granule exocytosis. J Immunol. 1994 Mar 1;152(5):2098–2104. [PubMed] [Google Scholar]
- Brock J. Lactoferrin: a multifunctional immunoregulatory protein? Immunol Today. 1995 Sep;16(9):417–419. doi: 10.1016/0167-5699(95)80016-6. [DOI] [PubMed] [Google Scholar]
- Evangelista V., Piccardoni P., White J. G., de Gaetano G., Cerletti C. Cathepsin G-dependent platelet stimulation by activated polymorphonuclear leukocytes and its inhibition by antiproteinases: role of P-selectin-mediated cell-cell adhesion. Blood. 1993 Jun 1;81(11):2947–2957. [PubMed] [Google Scholar]
- Evangelista V., Rajtar G., de Gaetano G., White J. G., Cerletti C. Platelet activation by fMLP-stimulated polymorphonuclear leukocytes: the activity of cathepsin G is not prevented by antiproteinases. Blood. 1991 Jun 1;77(11):2379–2388. [PubMed] [Google Scholar]
- Galandrini R., De Maria R., Piccoli M., Frati L., Santoni A. CD44 triggering enhances human NK cell cytotoxic functions. J Immunol. 1994 Nov 15;153(10):4399–4407. [PubMed] [Google Scholar]
- Gerard N. P., Gerard C. Construction and expression of a novel recombinant anaphylatoxin, C5a-N19, as a probe for the human C5a receptor. Biochemistry. 1990 Oct 2;29(39):9274–9281. doi: 10.1021/bi00491a024. [DOI] [PubMed] [Google Scholar]
- Gerber A. C., Carson J. H., Hadorn B. Partial purification and characterization of a chymotrypsin-like enzyme from human neutrophil leucocytes. Biochim Biophys Acta. 1974 Sep 11;364(1):103–112. doi: 10.1016/0005-2744(74)90137-5. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Eisenman J., Shanebeck K., Rauch C., Srinivasan S., Fung V., Beers C., Richardson J., Schoenborn M. A., Ahdieh M. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994 May 13;264(5161):965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- Hase-Yamazaki T., Aoki Y. Stimulation of human lymphocytes by cathepsin G. Cell Immunol. 1995 Jan;160(1):24–32. doi: 10.1016/0008-8749(95)80005-4. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Kuribayashi K., Kern D. E., Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981 May 28;291(5813):335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- Herberman R. R., Ortaldo J. R., Bonnard G. D. Augmentation by interferon of human natural and antibody-dependent cell-mediated cytotoxicity. Nature. 1979 Jan 18;277(5693):221–223. doi: 10.1038/277221a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Phillips J. H. Inhibitory MHC class I receptors on NK cells and T cells. Immunol Today. 1996 Feb;17(2):86–91. doi: 10.1016/0167-5699(96)80585-8. [DOI] [PubMed] [Google Scholar]
- Liao N. S., Bix M., Zijlstra M., Jaenisch R., Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991 Jul 12;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Hengartner H., Lichtenheld M. G. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- Santoni A., Gismondi A., Morrone S., Procopio A., Modesti A., Scarpa S., D'Orazi G., Piccoli M., Frati L. Rat natural killer cells synthesize fibronectin. Possible involvement in the cytotoxic function. J Immunol. 1989 Oct 1;143(7):2415–2421. [PubMed] [Google Scholar]
- Schmidt R. E., Bartley G., Levine H., Schlossman S. F., Ritz J. Functional characterization of LFA-1 antigens in the interaction of human NK clones and target cells. J Immunol. 1985 Aug;135(2):1020–1025. [PubMed] [Google Scholar]
- Schmidt W., Havemann K. Isolation of elastase-like and chymotrypsin-like neutral proteases from human granulocytes. Hoppe Seylers Z Physiol Chem. 1974 Sep;355(9):1077–1082. doi: 10.1515/bchm2.1974.355.2.1077. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Human cathepsin G. Catalytic and immunological properties. Biochem J. 1976 May 1;155(2):273–278. doi: 10.1042/bj1550273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Neutral proteinases of human spleen. Purification and criteria for homogeneity of elastase and cathepsin G. Biochem J. 1976 May 1;155(2):255–263. doi: 10.1042/bj1550255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Nakamura T., Koyanagi M., Kato K., Hashimoto Y., Yagita H., Okumura K. A murine very late activation antigen-like extracellular matrix receptor involved in CD2- and lymphocyte function-associated antigen-1-independent killer-target cell interaction. J Immunol. 1990 Dec 15;145(12):4371–4379. [PubMed] [Google Scholar]
- Timonen T., Gahmberg C. G., Patarroyo M. Participation of CD11a-c/CD18, CD2 and RGD-binding receptors in endogenous and interleukin-2-stimulated NK activity of CD3-negative large granular lymphocytes. Int J Cancer. 1990 Dec 15;46(6):1035–1040. doi: 10.1002/ijc.2910460615. [DOI] [PubMed] [Google Scholar]
- Timonen T., Patarroyo M., Gahmberg C. G. CD11a-c/CD18 and GP84 (LB-2) adhesion molecules on human large granular lymphocytes and their participation in natural killing. J Immunol. 1988 Aug 1;141(3):1041–1046. [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Nabholz M. Perforin-mediated target cell lysis by cytolytic T lymphocytes. Annu Rev Immunol. 1990;8:279–302. doi: 10.1146/annurev.iy.08.040190.001431. [DOI] [PubMed] [Google Scholar]
- Yamazaki T., Aoki Y. Cathepsin G binds to human lymphocytes. J Leukoc Biol. 1997 Jan;61(1):73–79. doi: 10.1002/jlb.61.1.73. [DOI] [PubMed] [Google Scholar]


