Abstract
Human immunodeficiency virus type 1 (HIV-1) virions bind to B cells in the peripheral blood and lymph nodes through interactions between CD21 on B cells and complement-complexed virions. B-cell-bound virions have been shown to be highly infectious, suggesting a unique mode of HIV-1 dissemination by B cells circulating between peripheral blood and lymphoid tissues. In order to investigate the relationship between B-cell-bound HIV-1 and viruses found in CD4+ T cells and in plasma, we examined the genetic relationships of HIV-1 found in the blood and lymph nodes of chronically infected patients with heteroduplex mobility and tracking assays and DNA sequence analysis. In samples from 13 of 15 patients examined, HIV-1 variants in peripheral blood-derived B cells were closely related to virus in CD4+ T cells and more divergent from virus in plasma. In samples from five chronically viremic patients for whom analyses were extended to include lymph node-derived HIV-1 isolates, B-cell-associated HIV-1 and CD4+-T-cell-associated HIV-1 in the lymph nodes were equivalent in their divergence from virus in peripheral blood-derived B cells and generally more distantly related to virus in peripheral blood-derived CD4+ T cells. These results indicates virologic cross talk between B cells and CD4+ T cells within the microenvironment of lymphoid tissues and, to a lesser extent, between cells in lymph nodes and the peripheral blood. These findings also indicate that most of the virus in plasma originates from cells other than CD4+ T cells in the peripheral blood and lymph nodes.
The selection and expansion of antigen-specific B cells in follicles of lymphoid tissues constitute hallmarks of the humoral immune response. Resident follicular dendritic cells (FDC), through their capacity to trap and present antigens, play a critical role in creating an intricate network for antigen-mediated interactions with B and T cells (16, 28). During human immunodeficiency virus (HIV) type 1 (HIV-1) infection, a large number of virions can be detected in the follicular areas of lymphoid tissues in the form of diffuse in situ hybridization signals that most likely represent virus-containing immune complexes (IC) trapped on FDC (1, 10). Furthermore, the FDC networks in germinal centers have been shown to account for the bulk of the HIV-1 RNA signal during ongoing viral replication (22) and are thought to represent an important source of residual “trapped” virus after plasma viremia is suppressed following the initiation of antiretroviral therapy (12).
It was recently demonstrated that lymphoid tissue-derived B cells and peripheral blood-derived B cells of HIV-1-infected viremic patients carry replication-competent virus on their surface (18). Similar to what has been proposed for FDC trapping of HIV-1 virions (14, 15), the mechanism of interaction between B cells and virus was found to involve the binding of HIV-containing IC to CD21 on the surface of the cells (18). It was also found that virus bound to B cells could efficiently infect activated CD4+ T cells through cell-cell contact (18). Recent in vitro studies have also demonstrated that HIV-1 in the form of IC bound to B cells is more stable and more efficiently passed to CD4+ T cells than its free-virion counterpart (13). Furthermore, considering that most interactions between lymphoid tissue-derived B cells and CD4+ T cells have been shown to occur at the border between follicular and T-cell-rich areas (9), B cells may constitute an important bridge between virus trapped on FDC and virus replicating in CD4+ T cells.
In the present study, we considered the relationship between HIV-1 bound to the surface of B cells and HIV-1 that constitutes plasma viremia by investigating the genotypic relationship between HIV-1 variants bound to the surface of B cells and virus replicating in CD4+ T cells and HIV-1 virions present in plasma. To this end, heteroduplex mobility assays (HMA) and heteroduplex tracking assays (HTA) were performed with cell- and plasma-associated HIV-1 derived from peripheral blood and cell-associated HIV-1 derived from lymph node biopsy samples. HMA is a rapid and sensitive approach for identifying genetic diversity, as HTA is for establishing genetic relationships among HIV-1 variants between and within individuals over time (6), as a function of the state of disease progression (5, 19) and immune stimulation (21), and upon interruption of effective antiretroviral therapy (2). Differences in virus fitness (25), cytopathogenicity (20), and viral populations isolated from various compartments (23, 31) have also been measured by using HTA. Here we used heteroduplex and sequencing analyses to demonstrate a close association between B-cell- and CD4+-T-cell-derived HIV-1 and to show that virus in CD4+ T cells in the blood or in lymph nodes is not the likely source of much of the virus in plasma.
MATERIALS AND METHODS
Study patients.
Fifteen patients chronically infected with HIV-1 were studied in accordance with protocols approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board. Of the 15 patients, 11 were chronically viremic due to antiretroviral drug resistance or nonadherence or were treatment naive (Table 1). The other four were recently viremic due to cessation of highly active antiretroviral therapy (HAART). Leukapheresis was performed on all patients studied, and excisional inguinal lymph node biopsies were performed on a subset of patients.
TABLE 1.
Profiles of HIV-1-infected patients and virus isolated from their blood
| Patient | Clinical statusa | CD4 count (cells/μl) | Plasma HIV-1 RNA (copies/ml)b | Plasma HIV-1 HMA resultc | T-cell-associated virus
|
B-cell-associated virus
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 RNA (copies/106 cells) | Days in cultured | p24, pg/mle | Coreceptor usagef | HIV-1 RNA (copies/106 cells) | Days in cultured | p24, pg/mle | Coreceptor usagef | |||||
| 1 | AIDS, nonadherence | 202 | 11,232 | Low | 26,000 | 3 | 365 (4) | R5 | 4,600 | 3 | 100 (3) | R5 |
| 2 | Asym, naive | 483 | 100,730 | High | 15,000 | 3-4 | 320 (3) | R5 | 4,700 | 3-4 | 120 (2) | R5 |
| 3 | AIDS, nonadherence | 987 | 62,743 | High | 35,000 | 3 | 100 (4) | R5 | 17,000 | 3 | 100 (3) | R5 |
| 4 | AIDS, nonadherence | 188 | 249,966 | Mod | NDg | 3 | 400 (4) | R5 | ND | 5 | 1,700 (7) | R5 |
| 5 | AIDS, nonadherence | 6 | 417,389 | High | 65,000 | 3-7 | 135 (5) | X4 (R5) | 6,500 | 4 | 115 (4) | X4 (R5) |
| 6 | AIDS, nonadherence | 92 | 16,989 | High | 5,400 | 2 | 4,000 (2) | X4 (R5) | 1,400 | 2-4 | 750 (2) | X4 (R5) |
| 7 | AIDS, failure | 108 | 133,822 | Low | 44,000 | 3 | 100 (3) | R5 | 17,000 | 3 | 100 (2) | R5 |
| 8 | AIDS, nonadherence | 136 | 47,489 | High | 12,000 | 3 | 200 (3) | X4 (R5) | 1,000 | 3 | 110 (3) | X4 (R5) |
| 9 | AIDS, nonadherence | 170 | 65,433 | Mod | 55,000 | 3 | 1,400 (4) | X4 | 13,000 | 3 | 100 (3) | X4 |
| 10 | AIDS, failure | 6 | 194,638 | Low | 6,700 | 4 | 188 (7) | X4, R5 | 8,300 | 4 | 253 (6) | X4, R5 |
| 11 | AIDS, failure | 53 | 244,174 | High | 51,000 | 3 | 200 (4) | X4 | 4,700 | 3 | 700 (2) | X4 |
| 12 | Asym, interruption | 316 | 166,143 | Mod | 42,000 | 4 | 1,316 (1) | R5 | 14,000 | 4 | 110 (1) | R5 |
| 13 | Asym, interruption | 1,573 | 109,850 | High | 1,800 | 4 | 250 (1) | R5 | 1,000 | 4 | 284 (1) | R5 |
| 14 | Asym, interruption | 797 | 241,785 | Low | ND | 3 | 100 (4) | R5 | ND | 6 | 250 (1) | R5 |
| 15 | Asym, interruption | 200 | 116,083 | Low | 19,000 | 3 | 760 (7) | R5 | 600 | 9 | 1,100 (2) | R5 |
Asym, aymptomatic; nonadherence, naive, failure, and interruption refer to antiretroviral therapy.
As measured by the Chiron bDNA assay, with a detection limit of 50 copies/ml.
Level of heterogeneity observed in HIV-1 RNA derived from 0.1 ml of plasma; low, 1 to 3 distinct bands; mod, 1 to 3 distinct bands with smears or 4 to 6 distinct bands; high, >6 bands with or without smears. Mod, moderate.
Coculture day on which supernatants were collected for HIV-1 RNA isolation.
Average release of p24 in supernatants of pooled wells (number of wells pooled).
Coreceptor usage of isolated virus was evaluated with U87-CD4 cell lines expressing CCR5 (R5) or CXCR4 (X4). R5 in parentheses indicates minor usage of CCR5.
ND, not determined.
Cell preparation and measurement of cell-associated HIV-1 RNA.
Highly enriched B cells were isolated from peripheral blood mononuclear cells (PBMC) and lymph node single-cell suspensions (LNMC) with a column-based negative-selection technique (StemCell Technologies, Vancouver, British Columbia, Canada) as described previously (18); B-cell marker CD20 or CD19 was expressed on greater than 95% and CD3 was expressed on less than 1% of the cells recovered. CD4+ T cells were enriched in parallel by immunomagnetic depletion of CD14+, CD8+, and CD19+ cells; CD19+-cell contamination represented less than 1% of the final cell preparation. To simplify data presentation, PBMC- and LNMC-derived B-cell compartments are referred to as PB-B and LN-B, respectively, whereas PBMC- and LNMC-derived CD4+ T-cell compartments are referred to as PB-T and LN-T, respectively.
Levels of cell-associated HIV-1 RNA were measured either by the nucleic acid sequence-based amplification technique (30) or by real-time reverse transcription (RT)-PCR. For RT-PCR, total cellular RNA was isolated from 1 × 106 to 5 × 106 cells with TRIzol reagent according to manufacturer specifications (Invitrogen, Carlsbad, Calif.). Synthesis of cDNA and real-time PCR were performed by using an iCycler (Bio-Rad, Hercules, Calif.) with a TaqMan EZ RT-PCR kit (Applied Biosystems, Foster City, Calif.) according to manufacturer specifications. PCR conditions consisted of denaturation at 95°C for 3 min followed by 45 cycles of 15 s at 95°C and 1 min at 60°C. The following primers and probe were used: 5′-TCTCTAGCAGTGGCGCCCGAACA-3′ (5′ primer), 5′-TCTCCTTCTAGCCTCCGCTAGTC-3′ (3′ primer), and 5′-6FAM-CAAGCCGAGTCCTGCGTCGAGAG-TAMRA-3′ (probe).
Culture conditions.
Replication-competent HIV-1 was amplified from patient cells by cocultivation with anti-CD3-activated PBMC from HIV-1-negative donors as described previously (18). Briefly, replicates of 0.2 × 106 to 1.0 × 106 patient-derived B-cell- or CD4+-T-cell-enriched populations were added to an equal number of 3-day-old CD3-stimulated, CD8-depleted PBMC from an HIV-negative donor. Coculture supernatants were tested periodically for the presence of virus by p24 antigen measurement with a commercial enzyme-linked immunosorbent assay (Beckman Coulter, Fullerton, Calif.). Controls for possible minor contamination of B-cell-associated HIV-1 in CD4+-T-cell-derived cocultures and vice versa included restricting analyses to samples in which substantial amounts of HIV-1 were recovered from short-term cultures (Table 1) and using the distinguishing feature that B-cell-associated HIV-1 is pronase sensitive whereas CD4+-T-cell-associated HIV-1 is not (18). Parallel cultures of pronase-treated and untreated cells resulted in no recovery of HIV-1 in pronase-treated B-cell cocultures and similar levels of HIV-1 in both pronase-treated and untreated CD4+-T-cell cocultures, indicating that there was no cross-contamination.
HMA and HTA.
HIV-1 env-specific RT-PCR-based HMA and HTA were performed as described previously (4, 7, 21) with replication-competent virus recovered from the supernatants of 2- to 9-day-old cocultures and from HIV-1 RNA isolated from 0.1 ml of plasma. Briefly, cDNA prepared from plasma or culture supernatants was subjected to nested PCR with primers ED5 and ED12 in the first round and DR7 and DR8 in the second round, generating a final amplification product of ∼650 bp spanning env regions C2 to V5. Parallel reactions were performed in the absence of reverse transcriptase to control for DNA contamination; RNA extracted from ACH-2 cells served as a negative control. 32P-labeled single-stranded probes were prepared from DR7-DR8 PCR products amplified from HIV-1 replicated in PBMC-derived B-cell cocultures. For all samples investigated, including plasma- and cell-derived sources of HIV-1, HMA and HTA patterns were confirmed by repeating the assays with separate aliquots of cDNA and, for a subset of patients, by repeating the assays with a separate vial of the same stock of cryopreserved PBMC. Controls for cross-contamination of samples being prepared for HTA included performing interpatient sample preparation alongside intrapatient sample preparation and comparing these HTA results to those obtained when patient samples were prepared independently.
Quantitative assessment of the relationship between the probe (PB-B-associated viral RNA) and target sequences by analysis of HTA banding patterns.
Densitomentric scans of autoradiographic gel images were performed on a Macintosh computer by using the public domain NIH Image program (developed at the National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). Background was subtracted from each lane, the total amount of signal in each lane of each gel was normalized to that in the probe-versus-PB-B lane, and gel length was normalized to adjust for slight differences in the mobility of the fastest band. The intensity of the signal at each pixel was subtracted from that in the PB-B lane, and the absolute differences were summed over the entire lane. One-half the amount of the absolute difference in intensity relative to the total normalized signal in the lane was reported.
Sequencing and phylogenetic analyses.
PCR amplification products were cloned into the plasmid vector PCR4TOPO (Invitrogen) and sequenced by Big-dye chain terminator chemistry (Applied Biosystems). Pools of 3 to 10 independent RNA preparations were used to make cDNA, and two to four series of nested PCR and cloning reactions were performed with the cDNA preparations. Sequencing reactions were performed with a minimum of 10 clones per sample that carried inserts of approximately 650 bp, as verified by agarose gel migration after excision of cloned fragments by restriction digestion. Sequences were manually edited by using SEQUENCHER (Gene Codes Corp., Ann Arbor, Mich). Nucleotide alignments were generated by using CLUSTAL W (29) and then edited and gap stripped by using MacClade (17). The best model of evolution for this data set was determined to be the HKY+Γ model by using the hierarchical likelihood ratio test as implemented in MODELTEST (24). This model was used in the construction of a neighbor-joining phylogenetic tree (26), which was then improved by branch swapping with the SPR algorithm in PAUP∗ (27) to obtain a maximum-likelihood (ML) tree (see Fig. 4). ML distances were then calculated for all possible pairs of sequences within the data set to obtain measures of nucleotide diversity (see Fig. 3).
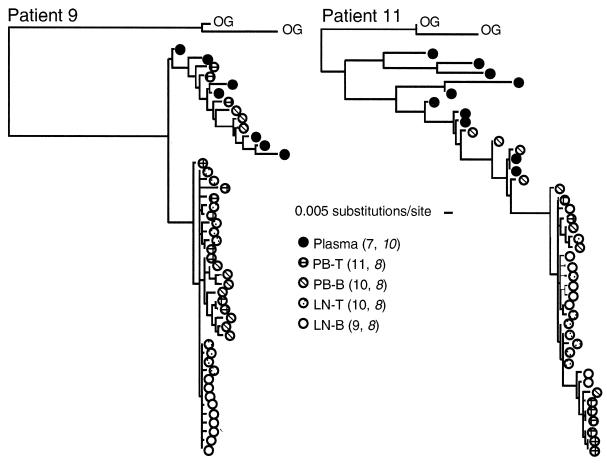
FIG. 4.
Phylogenetic analysis of HIV-1 in patients 9 and 11. ML phylogenetic trees (see Materials and Methods) depict the evolutionary relationships among viral sequences found in LN-B, LN-T, PB-T, PB-B, and plasma from each patient. The numbers of sequences analyzed for each sample are indicated in parentheses; those for patient 11 are shown in italic type. OG, outgroup sequences (GenBank accession numbers AF370890 and AF371037). The bar indicates substitutions per site calculated by the ML method (see Materials and Methods); hence, the calculated distances do not necessarily represent discrete integers of base substitutions.
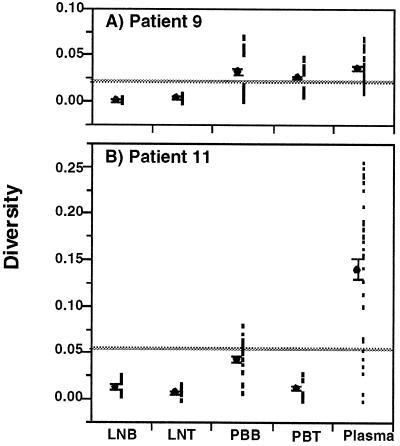
FIG. 3.
Diversity of HIV-1 env sequences in patients 9 and 11. Sequences (650 bp) encompassing the C2-V5 region of env were amplified from HIV-1 RNA taken from plasma and from viral supernatants derived from cocultures of PB-T (PBT), PB-B (PBB), LN-T (LNT), and LN-B (LNB). Measurements were made with 7 to 11 clones per sample (see the key on Fig. 4 for the exact number of clones analyzed for each sample); the total number of points per sample represented all pairwise comparisons of sequences. ML measurements of sequence diversity (see Materials and Methods) were determined for all sequence pairs within each virus population data set and plotted (filled squares). Means (filled circles) and standard errors of the means (error bars) are also shown offset to the left of each data plot. The mean of the combined data set is indicated by the horizontal gray line. Note that the means of the virus populations in PB-B and plasma in patient 9 are similar; however, the spread of data points for the PB-B population is distinctly bimodal, indicating that it is composed of two distinct populations of virus, each of which is relatively homogeneous (see the phylogenetic tree in Fig. 4).
Nucleotide sequence accession numbers.
The nucleotide sequences generated in this study have been deposited in GenBank under accession numbers AY077490 to AY077578.
RESULTS
Selection of patients and characterization of patient material.
In a previous study (18), replication-competent HIV-1 virions were shown to be present on PB-B of all patients whose plasma viral load was above 10,000 HIV-1 RNA copies per ml. Accordingly, patients in the present study were selected on the basis of plasma viral loads that would ensure representative HIV-1 samplings in plasma as well as cellular compartments (Table 1). HIV-1 env HMA were carried out to establish the level of diversity within each plasma sample. PCRs were initially carried out with cDNA representing 0.1 ml of plasma, indicating an initial range of 1,123 to 41,739 copies of HIV-1 RNA per reaction (Table 1). The low level of diversity detected by HMA in the plasma of patients 1, 7, 10, 14, and 15 suggested the presence of viral populations with a low level of genetic diversity in env or sampling of only one or a few viral templates in the PCR (Table 1). However, the large input of HIV-1 templates used for PCRs suggested that these populations did indeed have a low level of genetic diversity. Nonetheless, and to ensure the examination of large samplings of virus from these five patients, multiple reactions representing a total of 1.0 ml of plasma were performed and pooled for HTA. Again, the results revealed little heterogeneity detected by HMA and hence a low level of population diversity (data not shown).
Adequate sampling of the cellular compartments was shown by the large number of copies of input HIV-1 RNA present in each coculture well and the number of wells seeded and pooled for each sample (Table 1). Phenotypic relationships relative to coreceptor usage were established by exposing coculture supernatants to U87-CD4 cell lines expressing the chemokine receptor CCR5 or CXCR4. As illustrated in Table 1, pairs of HIV-1 isolates recovered from PB-B and PB-T fractions demonstrated identical profiles of coreceptor usage. Finally, while the values shown in Table 1 were measured with peripheral blood-derived cells, present (data not shown) and previous (18) observations indicated that lymph node-derived virus cultures contained approximately five times more HIV-1 RNA, indicating that representative sampling in this compartment was also achieved.
Genetic relationships between PB-B- and PB-T-associated HIV-1 variants in chronically infected patients.
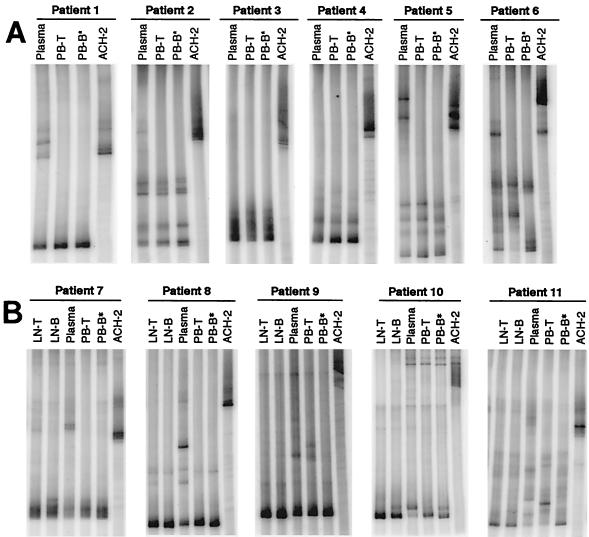
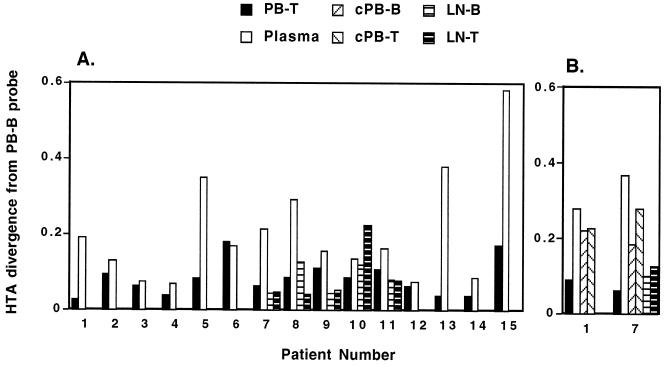
Genetic relationships between PB-B- and PB-T-associated replication-competent HIV-1 and plasma HIV-1 virions were investigated by performing HIV-1 env HTA with RNA isolated from coculture supernatants and plasma from the 11 chronically viremic patients described in Table 1. Fragments amplified from PB-B-associated virus were used as probes for comparison with HIV-1 RNAs isolated from PB-T and plasma. HTA performed with peripheral blood samples obtained from the 11 patients revealed that for all but 3 patients (patients 6, 9, and 11), PB-B and PB-T samples showed very similar HTA patterns (Fig. 1A and B). When quantitative differences between autoradiographic signals in the PB-B lane and other lanes were determined (Fig. 2A), these three patients showed the greatest distinctions between PB-B and PB-T specimens. In contrast, all but three patients (patients 2, 3, and 4) had plasma HTA patterns that were clearly distinct from the cell-derived patterns, despite certain bands being shared between cell-derived and plasma compartments. Only patients 6, 9, and 11 had PB-T and plasma samples that were both readily distinguishable from the PB-B probe. Taken together, these results suggest that in the peripheral blood of a majority of patients, the HIV-1 variants associated with B cells are more closely related to those in CD4+ T cells than to cell-free virus in plasma.
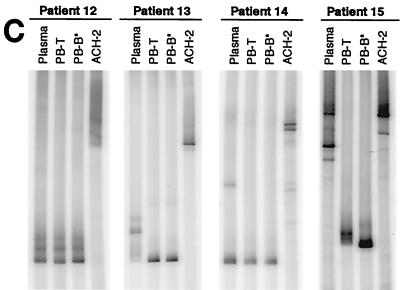
FIG. 1.
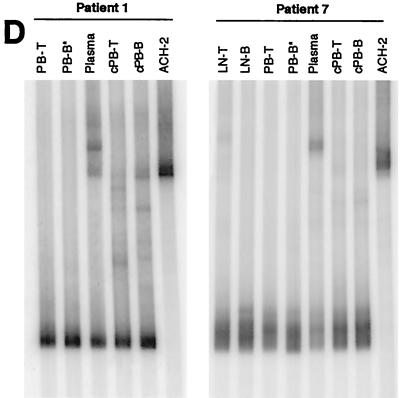
HTA of HIV-1 env sequences. Sequences (650 bp) encompassing the C2-V5 region of env were amplified from HIV-1 RNA taken from plasma and from viral supernatants derived from cocultures of PB-T and PB-B (A and C) as well as LN-T and LN-B (B). Samples were isolated from chronically viremic patients (A and B) and from recently viremic patients following discontinuation of HAART (C). HTA were extended to include HIV-1 RNA isolated directly ex vivo from PB-T and PB-B (cell associated; cPB-T and cPB-B) from patients 1 and 7 (D). PB-B-associated HIV-1 RT-PCR products were used as probes (asterisks denote probe lanes), and HIV-1 env amplified from ACH-2 cells was included as a control.
FIG. 2.
Quantitative image analysis of the HTA patterns shown in Fig. 1. As detailed in Materials and Methods, plots show the differences in band intensity distribution along each lane for a comparison of the PB-B lane to all other lanes. (A) Analysis of the HTA patterns in Fig. 1A to C. (B) Analysis of the HTA patterns in Fig. 1D.
It has been shown that viral rebound occurs within weeks of the cessation of highly active antiretroviral therapy (HAART) in a majority of HIV-1-infected patients, even in patients who have suppressed their plasma viremia for prolonged periods (3). B cells isolated from patients 4 to 5 weeks after the cessation of HAART have been shown to carry replication-competent HIV-1 on their surface (18). In the present study, the genetic relationships between PB-B- and PB-T-associated replication-competent HIV-1 as well as plasma-derived HIV-1 were evaluated by HTA for four patients who had recently discontinued HAART (Table 1). In three of the four patients (patients 12, 13, and 14), PB-B-associated HIV-1 variants were found to be similar to PB-T-associated variants, and in patient 12, the similarity extended to the plasma-derived virus HTA pattern (Fig. 1C). The PB-B-associated variants in patient 15 were divergent from the PB-T-associated variants yet the cell-derived variants were less divergent in relation to one another than to the plasma-derived variants (Fig. 2A). PB-B- and PB-T-associated HIV-1 variants appeared to be nearly absent from the plasma of patient 13, whereas in patient 14, they appeared to represent a subset of what was present in the plasma. Consistent with the results obtained for the chronically viremic patients, these data demonstrate a close association between virus bound to B cells and virus replicating in CD4+ T cells.
The higher degree of HIV-1 divergence observed between plasma and cell-derived samples might be explained by the fact that plasma contained both replication-competent variants and a greater excess of replication-defective variants than virus recovered from B and CD4+ T cells that were selected by their capacity to replicate in cocultures. We therefore performed HTA to compare HIV-1 RNA isolated from uncultured cells of patients 1 and 7 with HIV-1 RNA derived from their corresponding coculture supernatants (Fig. 1D). HTA patterns revealed that cell-associated HIV-1 RNA had marginally greater diversity than RNA from cultured virus, as evidenced by the slight increase in the number of minor slowly migrating bands in the lanes representing uncultured HIV-1 (Fig. 1D) as well as by quantitative image analysis (Fig. 2B). However, virus in plasma showed the highest degree of divergence relative to either replication-competent HIV-1 recovered from the PB-B and PB-T compartments or their respective uncultured HIV-1 counterparts. Taken together, these data suggest that cell-associated virus present on B cells and replicating in CD4+ T cells is largely distinct from free virus in plasma.
Close genetic relationships exist between HIV-1 variants found on B cells and in CD4+ T cells of lymphoid tissues.
In order to explore the possible origins of cell-associated and cell-free virus in the blood, we extended our genetic analysis to include HIV-1 variants present in inguinal lymph nodes by examining samples from biopsies performed on 5 of the 11 chronically viremic patients described in Table 1 (patients 7, 8, 9, 10, and 11). HTA were performed to compare replication-competent HIV-1 recovered from LN-B and LN-T sources and to compare HIV-1 variants found in the peripheral blood and lymph node compartments (Fig. 1B). Overall, the HTA patterns revealed that HIV-1 variants from LN-B had a high degree of similarity to viruses from the other cells and were divergent from the corresponding plasma-derived variants. However, some minor variants derived from LN-T were found in the plasma of patients 7 and 8. Furthermore, LN-B-derived virus patterns in patients 7, 8, and 10 shared similarities with PB-B- and PB-T-derived virus patterns that were distinct from LN-T-derived virus patterns. Greater differences between the sequences of viruses from PB-T in patient 9 and especially patient 11 and between the sequences of viruses from LN-T in patient 10 and the sequences of viruses from the other three cellular compartments were noted. Taken together, these data suggest that a close relationship exists between HIV-1 isolates derived from LN-B and LN-T, and there were some indications that virus on B cells in the lymph node compartment is more closely related to virus obtained from peripheral blood B and CD4+ T cells than to virus in their CD4+-T-cell counterparts in the lymph node compartment.
Sequence analysis confirms virologic cross talk between B and CD4+ T cells in lymphoid tissues.
Viral env gene sequencing was performed with plasma, PB-B, PB-T, LN-B, and LN-T samples from the two patients who had the most contrasting HTA patterns, namely, patients 9 and 11. For both patients, 7 to 11 clones were analyzed for each cell compartment and plasma sample. Consistent with the HMA and HTA results, the sequence diversity was greater within patient 11 than within patient 9, and plasma was the most diverse compartment in both patients (Fig. 3). It should be noted that while the overall diversities of sequences in the PB-B and plasma samples were similar for patient 9, the distribution of data points for the PB-B sample clustered into two distinct yet relatively homogeneous populations of virus. Conversely, the least heterogeneity was found in the LN-B and LN-T samples from both patients (Fig. 3).
Relationships between viruses associated with different compartments were evaluated by phylogenetic analysis (Fig. 4). A close relationship was found between LN-B- and LN-T-derived viruses in both patients, and in neither patient was there evidence in plasma of virus like that in the lymph nodes. Each patient had a cluster of closely related viruses derived from cultures of lymph node and peripheral blood cells (Fig. 4, bottom part of each tree). A more diverse population of viruses found in plasma clustered with another subset of PB-B-derived viruses in both patients. This cluster also included PB-T-derived virus in patient 9.
DISCUSSION
The present study demonstrates that there is a close relationship between virus circulating on B cells and virus replicating in CD4+ T cells of HIV-1-infected patients and establishes the presence of virologic cross talk between cell-associated viruses in peripheral blood and lymph nodes. We simultaneously examined three to seven virus populations from HIV-1-infected patients, including virus cultivated in vitro from separated CD4+ T and B cells from the peripheral blood and lymph nodes, viral RNA from plasma, and cell-associated viral RNA from peripheral blood B and CD4+ T cells. We established that the replication-competent HIV-1 virions bound to B cells in the peripheral blood and lymph nodes of chronically infected patients are closely related to virus variants found in CD4+ T cells in the same lymphoid compartments. This finding suggests the presence of virologic cross talk between B and CD4+ T cells in the microenvironment of lymphoid tissues, with the bulk of the virus on B cells loaded locally. This finding is consistent with recent findings showing a close genetic relationship between HIV-1 trapped on FDC and virus isolated from CD4+ T cells in splenic white pulp (M.-J. Dumaurier, R. Cheynier, and S. Wain-Hobson, Abstr. 7th Conf. Retroviruses Opportunistic Infect., abstr. 444, 2000).
The origin of virus present in plasma, however, is more complex. For all patients studied, the diversity of virus populations within the plasma of a given patient was at least as great as the diversity within the cells of the same patient. In 12 of 15 patients, plasma-derived virus was substantially more diverse than cell-derived virus populations. Furthermore, the bulk of virus in plasma was genetically distinct from that in any of the B-cell or CD4+-T-cell populations evaluated. Sequence analysis of viruses from two patients did show that some of the PB-B-associated virus clustered with virus found in plasma, suggesting that virus in plasma can also adhere to B cells or that this subset of B cells originated from the same compartment as that from which the plasma-derived virus originated. However, only in patient 9 did PB-T-derived virus cluster with plasma-derived virus, and in neither patient did LN-T-derived virus cluster with that found in plasma. Although there was no bootstrap support for the clusters seen in the trees, clustering was significantly nonrandom (Fig. 5). Hence, the bulk of virus in plasma is derived from a cellular source outside the peripheral blood and lymph nodes, such as tissue macrophages (13, 22, 25; G. S. Laco, D. C. Nickle, S. J. Brodie, A. J. Melvin, K. M. Mohan, A. G. Rodrigo, L. M. Frenkel, and J. I. Mullins, submitted for publication).
FIG. 5.
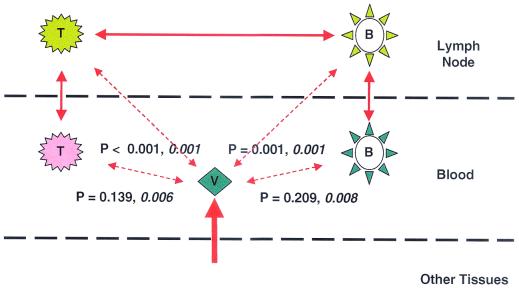
Schematic representation of the relationships between viruses in cellular compartments and plasma from patients 9 and 11. Virus populations from each of the four cellular compartments and plasma (V) are represented schematically. Levels of relatedness between pairs of compartments, as indicated by bidirectional arrows, were determined statistically by establishing the likelihood that the two populations being compared were the same. When P values were significant (indicating that the populations being compared were significantly different) for either both patients or one of the two patients, the arrows are broken and the P values are shown (values for patient 11 are shown in italic type). The thickness of the lines represents the degree of virus trafficking suggested by the phylogenetic and statistical analyses shown. Since little of the cell-associated virus is closely related to virus in plasma, much of the virus in plasma is derived from other tissues (thickest line).
In three patients (patients 7, 8, and 10), HTA showed a pattern of minor variants in common among PB-B, PB-T, and LN-B (with a greater divergence in LN-T; Fig. 1B), indicating some cross talk involving B-cell compartments of the lymph nodes and peripheral blood. The overall relationships that we have noted are summarized schematically in Fig. 5.
One issue that remains unresolved is whether virions deposited on FDC and B cells are part of a cycle that feeds from plasma viremia in the peripheral circulation or from local CD4+ T cells that are productively replicating HIV-1, although there are indications that FDC-bound virions are of local origin (Dumaurier et al., Abstr. 7th Conf. Retroviruses Opportunistic Infect.). Nonetheless, ex vivo studies clearly show that FDC and B cells can potentially contribute to ongoing viral replication by serving as extracellular reservoirs of HIV-1 (11, 18). HIV-1 propagation through the cell-cell route has been shown to be much more effective than that through virus-cell contact (8), and the IC-based interactions that are thought to mediate the binding of HIV-1 to FDC and B cells (15) have been shown to increase the infectious capacity of HIV-1 in vitro compared to that of non-cell-associated virions (13). In light of these findings and the basic features that distinguish B cells from FDC, FDC may be considered a long-lived stationary repository of HIV-1 virions, whereas B cells may represent a more labile yet more mobile repository of HIV-1.
Acknowledgments
Angela Malaspina and Susan Moir contributed equally to this work.
We thank Patricia Walsh for editorial assistance and Julie Metcalf for coordinating patient visits and samplings. We are indebted to the patients for their willingness to participate in these studies.
This work was supported in part by grants from the U.S. Public Health Service to J.I.M. and to the University of Washington Center for AIDS Research.
REFERENCES
- 1.Burton, G. F., A. Masuda, S. L. Heath, B. A. Smith, J. G. Tew, and A. K. Szakal. 1997. Follicular dendritic cells (FDC) in retroviral infection: host/pathogen perspectives. Immunol. Rev. 156:185-197. [DOI] [PubMed] [Google Scholar]
- 2.Chun, T. W., R. T. Davey, Jr., M. Ostrowski, J. Shawn Justement, D. Engel, J. I. Mullins, and A. S. Fauci. 2000. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat. Med. 6:757-761. [DOI] [PubMed] [Google Scholar]
- 3.Davey, R. T., Jr., N. Bhat, C. Yoder, T. W. Chun, J. A. Metcalf, R. Dewar, V. Natarajan, R. A. Lempicki, J. W. Adelsberger, K. D. Miller, J. A. Kovacs, M. A. Polis, R. E. Walker, J. Falloon, H. Masur, D. Gee, M. Baseler, D. S. Dimitrov, A. S. Fauci, and H. C. Lane. 1999. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. USA 96:15109-15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delwart, E. L., B. Herring, A. G. Rodrigo, and J. I. Mullins. 1995. Genetic subtyping of human immunodeficiency virus using a heteroduplex mobility assay. PCR Methods Appl. 4:S202-S216. [DOI] [PubMed] [Google Scholar]
- 5.Delwart, E. L., and C. J. Gordon. 1997. Tracking changes in HIV-1 envelope quasispecies using DNA heteroduplex analysis. Methods 12:348-354. [DOI] [PubMed] [Google Scholar]
- 6.Delwart, E. L., H. W. Sheppard, B. D. Walker, J. Goudsmit, and J. I. Mullins. 1994. Human immunodeficiency virus type 1 evolution in vivo tracked by DNA heteroduplex mobility assays. J. Virol. 68:6672-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delwart, E. L., E. G. Shpaer, J. Louwagie, F. E. McCutchan, M. Grez, H. Rubsamen-Waigmann, and J. I. Mullins. 1993. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science 262:1257-1261. [DOI] [PubMed] [Google Scholar]
- 8.Dimitrov, D. S., R. L. Willey, H. Sato, L. J. Chang, R. Blumenthal, and M. A. Martin. 1993. Quantitation of human immunodeficiency virus type 1 infection kinetics. J. Virol. 67:2182-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garside, P., E. Ingulli, R. R. Merica, J. G. Johnson, R. J. Noelle, and M. K. Jenkins. 1998. Visualization of specific B and T lymphocyte interactions in the lymph node. Science 281:96-99. [DOI] [PubMed] [Google Scholar]
- 10.Haase, A. T. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17:625-656. [DOI] [PubMed] [Google Scholar]
- 11.Heath, S. L., J. G. Tew, A. K. Szakal, and G. F. Burton. 1995. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature 377:740-744. [DOI] [PubMed] [Google Scholar]
- 12.Hlavacek, W. S., C. Wofsy, and A. S. Perelson. 1999. Dissociation of HIV-1 from follicular dendritic cells during HAART: mathematical analysis. Proc. Natl. Acad. Sci. USA 96:14681-14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakubik, J. J., M. Saifuddin, D. M. Takefman, and G. T. Spear. 2000. Immune complexes containing human immunodeficiency virus type 1 primary isolates bind to lymphoid tissue B lymphocytes and are infectious for T lymphocytes. J. Virol. 74:552-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joling, P., L. J. Bakker, J. A. Van Strijp, T. Meerloo, L. de Graaf, M. E. Dekker, J. Goudsmit, J. Verhoef, and H. J. Schuurman. 1993. Binding of human immunodeficiency virus type-1 to follicular dendritic cells in vitro is complement dependent. J. Immunol. 150:1065-1073. [PubMed] [Google Scholar]
- 15.Kacani, L., W. M. Prodinger, G. M. Sprinzl, M. G. Schwendinger, M. Spruth, H. Stoiber, S. Dopper, S. Steinhuber, F. Steindl, and M. P. Dierich. 2000. Detachment of human immunodeficiency virus type 1 from germinal centers by blocking complement receptor type 2. J. Virol. 74:7997-8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koopman, G., and S. T. Pals. 1992. Cellular interactions in the germinal center: role of adhesion receptors and significance for the pathogenesis of AIDS and malignant lymphoma. Immunol. Rev. 126:21-45. [DOI] [PubMed] [Google Scholar]
- 17.Maddison, D. R., and W. R. Maddison. 1992. MacClade—analysis of phylogeny and character evolution—version 3. Sinauer Associates, Inc., Sunderland, Mass.
- 18.Moir, S., A. Malaspina, Y. Li, T. W. Chun, T. Lowe, J. Adelsberger, M. Baseler, L. A. Ehler, S. Liu, R. T. Davey, Jr., J. A. Mican, and A. S. Fauci. 2000. B cells of HIV-1-infected patients bind virions through CD21-complement interactions and transmit infectious virus to activated T cells. J. Exp. Med. 192:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson, J. A., F. Baribaud, T. Edwards, and R. Swanstrom. 2000. Patterns of changes in human immunodeficiency virus type 1 V3 sequence populations late in infection. J. Virol. 74:8494-8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson, J. A., S. A. Fiscus, and R. Swanstrom. 1997. Evolutionary variants of the human immunodeficiency virus type 1 V3 region characterized by using a heteroduplex tracking assay. J. Virol. 71:8750-8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrowski, M. A., D. C. Krakauer, Y. Li, S. J. Justement, G. Learn, L. A. Ehler, S. K. Stanley, M. Nowak, and A. S. Fauci. 1998. Effect of immune activation on the dynamics of human immunodeficiency virus replication and on the distribution of viral quasispecies. J. Virol. 72:7772-7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pantaleo, G., C. Graziosi, J. F. Demarest, L. Butini, M. Montroni, C. H. Fox, J. M. Orenstein, D. P. Kotler, and A. S. Fauci. 1993. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 362:355-358. [DOI] [PubMed] [Google Scholar]
- 23.Ping, L. H., M. S. Cohen, I. Hoffman, P. Vernazza, F. Seillier-Moiseiwitsch, H. Chakraborty, P. Kazembe, D. Zimba, M. Maida, S. A. Fiscus, J. J. Eron, R. Swanstrom, and J. A. Nelson. 2000. Effects of genital tract inflammation on human immunodeficiency virus type 1 V3 populations in blood and semen. J. Virol. 74:8946-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Posada, D., and K. A. Crandall. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817-818. [DOI] [PubMed] [Google Scholar]
- 25.Quinones-Mateu, M. E., S. C. Ball, A. J. Marozsan, V. S. Torre, J. L. Albright, G. Vanham, G. van Der Groen, R. L. Colebunders, and E. J. Arts. 2000. A dual infection/competition assay shows a correlation between ex vivo human immunodeficiency virus type 1 fitness and disease progression. J. Virol. 74:9222-9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 27.Swofford, D. L. 1999. PAUP∗: phylogenetic analysis using parsimony, edition 4.0. Sinauer Assoicates, Sunderland, Mass.
- 28.Tew, J. G., and T. E. Mandel. 1979. Prolonged antigen half-life in the lymphoid follicles of specifically immunized mice. Immunology 37:69-76. [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Gemen, B., R. van Beuningen, A. Nabbe, D. van Strijp, S. Jurriaans, P. Lens, and T. Kievits. 1994. A one-tube quantitative HIV-1 RNA NASBA nucleic acid amplification assay using electrochemiluminescent (ECL) labelled probes. J. Virol. Methods 49:157-167. [DOI] [PubMed] [Google Scholar]
- 31.Zhu, T., N. Wang, A. Carr, D. S. Nam, R. Moor-Jankowski, D. A. Cooper, and D. D. Ho. 1996. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J. Virol. 70:3098-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]