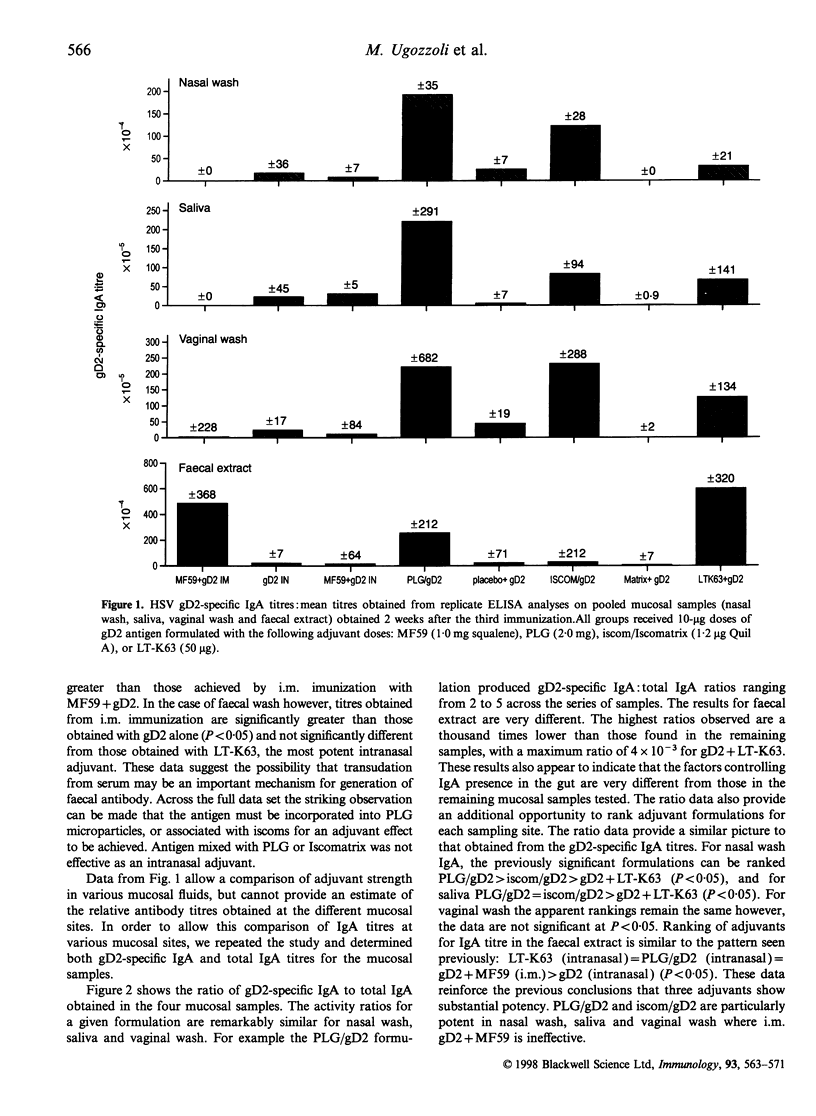
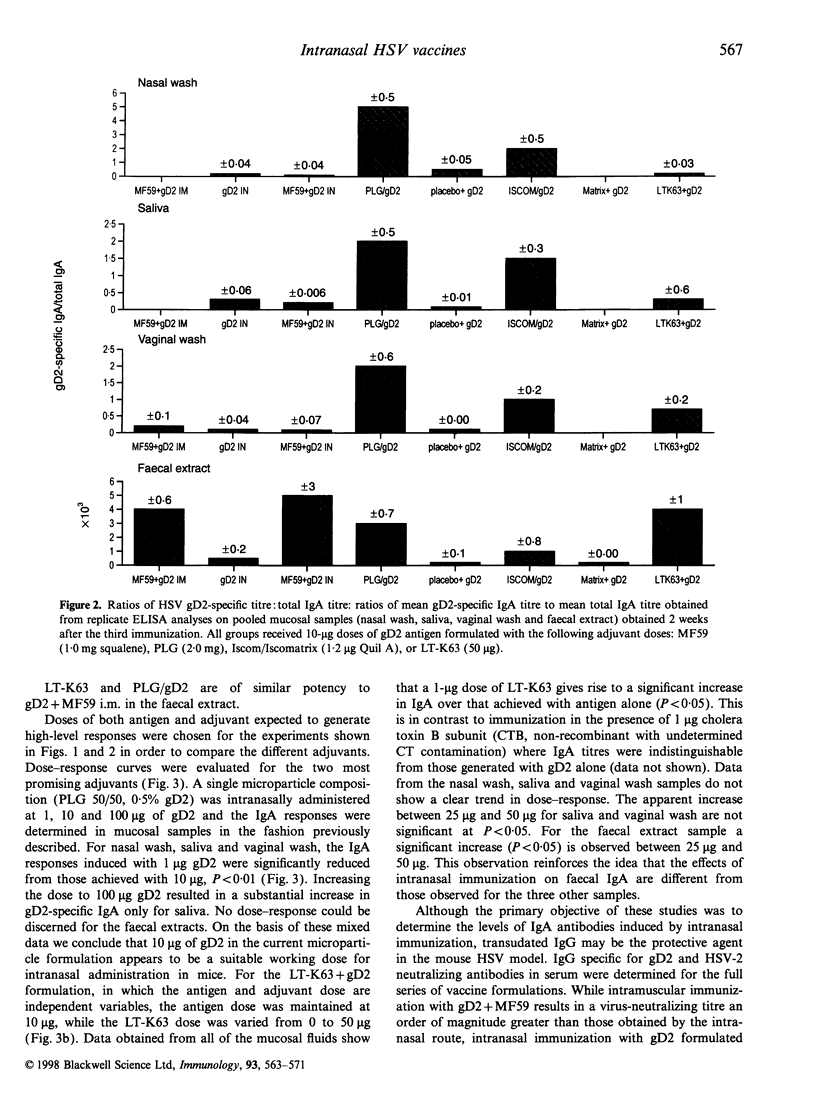
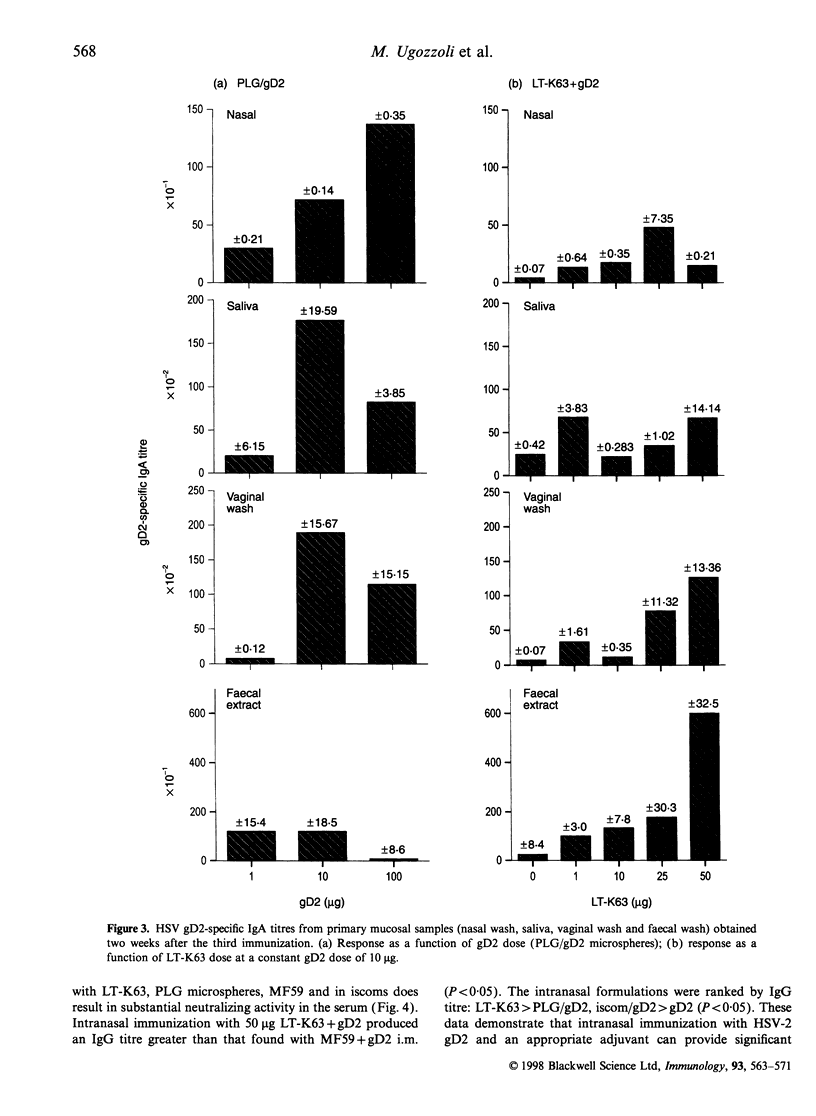
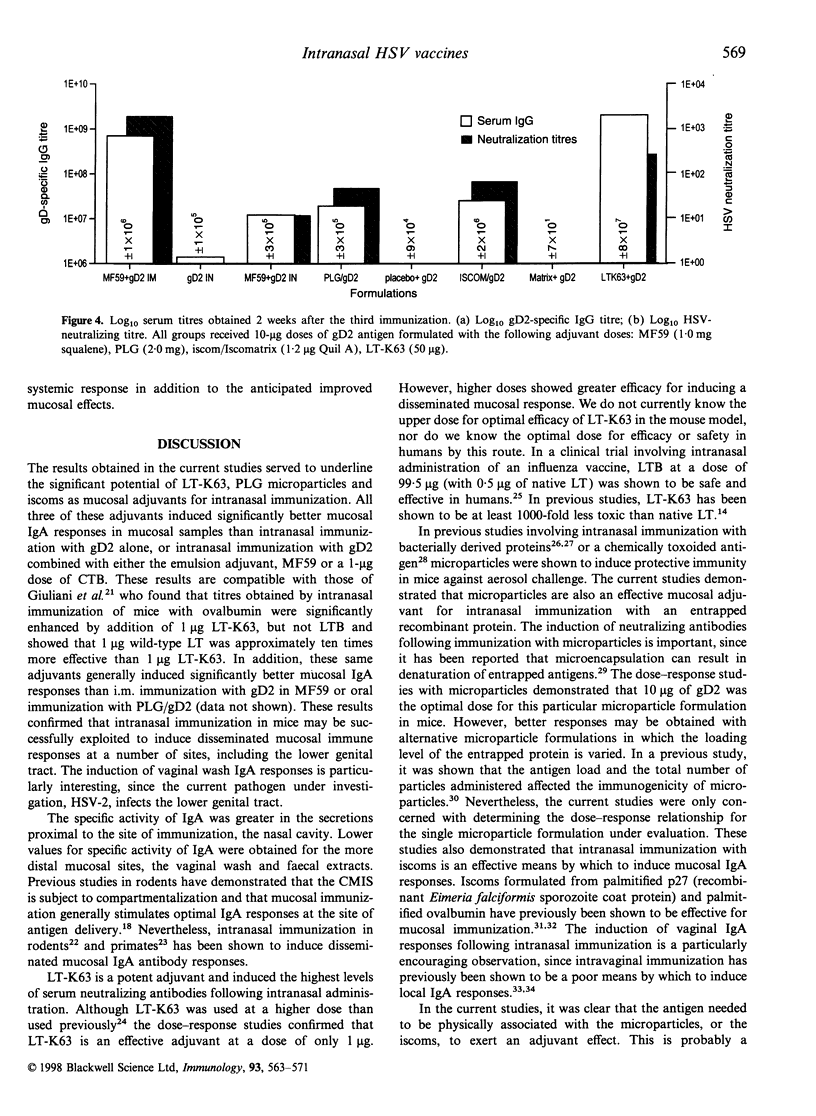
Abstract
Mucosal immunization offers the potential for inducing IgA antibody responses in the vagina, the site of infection for many viruses, including herpes simplex type 2 (HSV-2). To investigate this possibility, mice were immunized intranasally with 10 micrograms glycoprotein D2 (gD2) from HSV combined with a series of adjuvants of proven efficacy; the oil in water emulsion MF59, poly(D,L-lactide-co-glycolide) microparticles (PLG) (encapsulated or co-administered), immune-stimulating complexes (iscoms) (incorporated or co-administered with iscomatrix) and the genetically detoxified enterotoxin from Escherichia coli, LT-K63. Encapsulation of gD2 into PLG microparticles, incorporation of gD2 into iscoms and co-administration of gD2 with LT-K63 induced mucosal IgA antibody responses (nasal wash, saliva and vaginal wash) which were greater than those induced by intramuscular administration of gD2 with MF59. Intranasal immunization with these formulations also induced substantial levels of serum IgG and neutralizing antibodies. These studies demonstrated that intranasal immunization with potent adjuvants is an effective means to induce mucosal antibody responses, even in the lower genital tract.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengtsson K. L., Sjölander A. Adjuvant activity of iscoms; effect of ratio and co-incorporation of antigen and adjuvant. Vaccine. 1996 Jun;14(8):753–760. doi: 10.1016/0264-410x(95)00253-w. [DOI] [PubMed] [Google Scholar]
- Cahill E. S., O'Hagan D. T., Illum L., Barnard A., Mills K. H., Redhead K. Immune responses and protection against Bordetella pertussis infection after intranasal immunization of mice with filamentous haemagglutinin in solution or incorporated in biodegradable microparticles. Vaccine. 1995 Apr;13(5):455–462. doi: 10.1016/0264-410x(94)00008-b. [DOI] [PubMed] [Google Scholar]
- Corey L., Holmes K. K. Genital herpes simplex virus infections: current concepts in diagnosis, therapy, and prevention. Ann Intern Med. 1983 Jun;98(6):973–983. doi: 10.7326/0003-4819-98-6-973. [DOI] [PubMed] [Google Scholar]
- Di Tommaso A., Saletti G., Pizza M., Rappuoli R., Dougan G., Abrignani S., Douce G., De Magistris M. T. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996 Mar;64(3):974–979. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge J. H., Staas J. K., Meulbroek J. A., Tice T. R., Gilley R. M. Biodegradable and biocompatible poly(DL-lactide-co-glycolide) microspheres as an adjuvant for staphylococcal enterotoxin B toxoid which enhances the level of toxin-neutralizing antibodies. Infect Immun. 1991 Sep;59(9):2978–2986. doi: 10.1128/iai.59.9.2978-2986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneberg B., Kendall D., Amerongen H. M., Apter F. M., Kraehenbuhl J. P., Neutra M. R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994 Jan;62(1):15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashigucci K., Ogawa H., Ishidate T., Yamashita R., Kamiya H., Watanabe K., Hattori N., Sato T., Suzuki Y., Nagamine T. Antibody responses in volunteers induced by nasal influenza vaccine combined with Escherichia coli heat-labile enterotoxin B subunit containing a trace amount of the holotoxin. Vaccine. 1996 Feb;14(2):113–119. doi: 10.1016/0264-410x(95)00174-y. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Fujihashi K., Kiyono H., McGhee J. R. Luminometry: a novel bioluminescent immunoassay enhances the quantitation of mucosal and systemic antibody responses. J Immunol Methods. 1996 Apr 19;190(2):189–197. doi: 10.1016/0022-1759(95)00276-6. [DOI] [PubMed] [Google Scholar]
- Jeffery H., Davis S. S., O'Hagan D. T. The preparation and characterization of poly(lactide-co-glycolide) microparticles. II. The entrapment of a model protein using a (water-in-oil)-in-water emulsion solvent evaporation technique. Pharm Res. 1993 Mar;10(3):362–368. doi: 10.1023/a:1018980020506. [DOI] [PubMed] [Google Scholar]
- Johnson R. M., Spear P. G. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989 Feb;63(2):819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanji M., Laurent F., Péry P. Immune responses and protective effect in mice vaccinated orally with surface sporozoite protein of Eimeria falciformis in ISCOMs. Vaccine. 1994 Jul;12(9):798–804. doi: 10.1016/0264-410x(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Langenberg A. G., Burke R. L., Adair S. F., Sekulovich R., Tigges M., Dekker C. L., Corey L. A recombinant glycoprotein vaccine for herpes simplex virus type 2: safety and immunogenicity [corrected]. Ann Intern Med. 1995 Jun 15;122(12):889–898. doi: 10.7326/0003-4819-122-12-199506150-00001. [DOI] [PubMed] [Google Scholar]
- Lövgren K., Lindmark J., Pipkorn R., Morein B. Antigenic presentation of small molecules and peptides conjugated to a preformed iscom as carrier. J Immunol Methods. 1987 Apr 2;98(1):137–143. doi: 10.1016/0022-1759(87)90447-9. [DOI] [PubMed] [Google Scholar]
- Morein B., Sundquist B., Höglund S., Dalsgaard K., Osterhaus A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. 1984 Mar 29-Apr 4Nature. 308(5958):457–460. doi: 10.1038/308457a0. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Donachie A. M., Reid G., Jarrett O. Immune-stimulating complexes containing Quil A and protein antigen prime class I MHC-restricted T lymphocytes in vivo and are immunogenic by the oral route. Immunology. 1991 Mar;72(3):317–322. [PMC free article] [PubMed] [Google Scholar]
- Nahmias A. J., Lee F. K., Beckman-Nahmias S. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand J Infect Dis Suppl. 1990;69:19–36. [PubMed] [Google Scholar]
- O'Hagan D. T., Rafferty D., McKeating J. A., Illum L. Vaginal immunization of rats with a synthetic peptide from human immunodeficiency virus envelope glycoprotein. J Gen Virol. 1992 Aug;73(Pt 8):2141–2145. doi: 10.1099/0022-1317-73-8-2141. [DOI] [PubMed] [Google Scholar]
- O'Hagan D. T., Rahman D., McGee J. P., Jeffery H., Davies M. C., Williams P., Davis S. S., Challacombe S. J. Biodegradable microparticles as controlled release antigen delivery systems. Immunology. 1991 Jun;73(2):239–242. [PMC free article] [PubMed] [Google Scholar]
- Pizza M., Domenighini M., Hol W., Giannelli V., Fontana M. R., Giuliani M. M., Magagnoli C., Peppoloni S., Manetti R., Rappuoli R. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol Microbiol. 1994 Oct;14(1):51–60. doi: 10.1111/j.1365-2958.1994.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Renegar K. B., Small P. A., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991 Mar 15;146(6):1972–1978. [PubMed] [Google Scholar]
- Sanchez-Pescador L., Burke R. L., Ott G., Van Nest G. The effect of adjuvants on the efficacy of a recombinant herpes simplex virus glycoprotein vaccine. J Immunol. 1988 Sep 1;141(5):1720–1727. [PubMed] [Google Scholar]
- Shahin R., Leef M., Eldridge J., Hudson M., Gilley R. Adjuvanticity and protective immunity elicited by Bordetella pertussis antigens encapsulated in poly(DL-lactide-co-glycolide) microspheres. Infect Immun. 1995 Apr;63(4):1195–1200. doi: 10.1128/iai.63.4.1195-1200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuve L. L., Brown-Shimer S., Pachl C., Najarian R., Dina D., Burke R. L. Structure and expression of the herpes simplex virus type 2 glycoprotein gB gene. J Virol. 1987 Feb;61(2):326–335. doi: 10.1128/jvi.61.2.326-335.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar M. A., Parr E. L., Bozzola J. J., Parr M. B. Secretory immune responses in the mouse vagina after parenteral or intravaginal immunization with an immunostimulating complex (ISCOM). Vaccine. 1991 Feb;9(2):129–133. doi: 10.1016/0264-410x(91)90269-c. [DOI] [PubMed] [Google Scholar]
- Uchida T., Martin S., Foster T. P., Wardley R. C., Grimm S. Dose and load studies for subcutaneous and oral delivery of poly(lactide-co-glycolide) microspheres containing ovalbumin. Pharm Res. 1994 Jul;11(7):1009–1015. doi: 10.1023/a:1018987404751. [DOI] [PubMed] [Google Scholar]
- Winner L., 3rd, Mack J., Weltzin R., Mekalanos J. J., Kraehenbuhl J. P., Neutra M. R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991 Mar;59(3):977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. Y., Russell M. W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993 Jan;61(1):314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Rill W. L., Malli R., Hewetson J., Naseem H., Tammariello R., Kende M. Intranasal stimulation of long-lasting immunity against aerosol ricin challenge with ricin toxoid vaccine encapsulated in polymeric microspheres. Vaccine. 1996 Aug;14(11):1031–1038. doi: 10.1016/0264-410x(96)00063-1. [DOI] [PubMed] [Google Scholar]


