Abstract
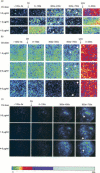
A series of permeability thresholds to Ca2+ metabolites and macromolecules, occurring at different times when cells are attacked by complement, has been established by imaging HeLa cells transiently expressing a recombinant cytosolic fusion protein of firefly luciferase and aequorin (luciferase-aequorin) to measure changes in ATP and cytosolic free Ca2+. Nuclear fluorescence of propidium was used as a measure of permeability to small molecules, and luciferase activity imaged to assess lysis. The rise in cytosolic free Ca2+ observed after C9 attack preceded by at least 60 s both the increase in propidium fluorescence, measured in single cells, and the decrease in ATP monitored by luciferase light emission. These effects were dependent on the concentration of C9. At concentrations of C9 up to 4 micrograms/ml no loss of luciferase-aequorin protein was detected at the end of the experiment. Thus the membrane integrity of the cells remained intact, even though the cells were permeable to propidium. These results confirmed our earlier observations that propidium permeability in cells attacked by complement was not a reliable measure of cell death. They also show that it is vital to take account of cellular heterogeneity if the mechanisms by which cells respond to membrane pore former attack are to be correctly interpreted.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badminton M. N., Kendall J. M., Sala-Newby G., Campbell A. K. Nucleoplasmin-targeted aequorin provides evidence for a nuclear calcium barrier. Exp Cell Res. 1995 Jan;216(1):236–243. doi: 10.1006/excr.1995.1030. [DOI] [PubMed] [Google Scholar]
- Campbell A. K., Daw R. A., Hallett M. B., Luzio J. P. Direct measurement of the increase in intracellular free calcium ion concentration in response to the action of complement. Biochem J. 1981 Feb 15;194(2):551–560. doi: 10.1042/bj1940551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan M. H., Dewey T. G. Steady state kinetics of ATP synthesis and hydrolysis coupled calcium transport catalyzed by the reconstituted sarcoplasmic reticulum ATPase. J Biol Chem. 1985 May 25;260(10):6147–6152. [PubMed] [Google Scholar]
- Gandelman O., Allue I., Bowers K., Cobbold P. Cytoplasmic factors that affect the intensity and stability of bioluminescence from firefly luciferase in living mammalian cells. J Biolumin Chemilumin. 1994 Nov-Dec;9(6):363–371. doi: 10.1002/bio.1170090603. [DOI] [PubMed] [Google Scholar]
- Hallett M. B., Luzio J. P., Campbell A. K. Stimulation of Ca2+-dependent chemiluminescence in rat polymorphonuclear leucocytes by polystyrene beads and the non-lytic action of complement. Immunology. 1981 Nov;44(3):569–576. [PMC free article] [PubMed] [Google Scholar]
- Hallett M. B., Newby A. C., Luzio J. P., Campbell A. K. Rapid stimulation of chemiluminescence in rat polymorphonuclear leucocytes caused by anti-cell antibody plus complement. Biochem Soc Trans. 1980 Dec;8(6):723–725. doi: 10.1042/bst0080723. [DOI] [PubMed] [Google Scholar]
- Kendall J. M., Badminton M. N., Sala-Newby G. B., Wilkinson G. W., Campbell A. K. Agonist-stimulated free calcium in subcellular compartments. Delivery of recombinant aequorin to organelles using a replication deficient adenovirus vector. Cell Calcium. 1996 Feb;19(2):133–142. doi: 10.1016/s0143-4160(96)90082-2. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J. The control of homologous lysis. Immunol Today. 1991 Sep;12(9):312–315. doi: 10.1016/0167-5699(91)90005-E. [DOI] [PubMed] [Google Scholar]
- Laffafian I., Davies E. V., Campbell A. K., Hallett M. B. Complement component C9-dependent cytosolic free Ca2+ rise and recovery in neutrophils. Cell Calcium. 1995 Apr;17(4):279–286. doi: 10.1016/0143-4160(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Laiho K. U., Trump B. F. The relationship between cell viability and changes in mitochondrial ultrastructure, cellular ATP, ion and water content following injury of Ehrlich ascites tumor cells. Virchows Arch B Cell Pathol. 1974;15(4):267–277. doi: 10.1007/BF02889343. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Campbell A. K. The recovery of human polymorphonuclear leucocytes from sublytic complement attack is mediated by changes in intracellular free calcium. Biochem J. 1985 Oct 1;231(1):205–208. doi: 10.1042/bj2310205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P. Non-lethal complement-membrane attack on human neutrophils: transient cell swelling and metabolic depletion. Immunology. 1988 Jan;63(1):71–77. [PMC free article] [PubMed] [Google Scholar]
- Newsholme P., Adogu A. A., Soos M. A., Hales C. N. Complement-induced Ca2+ influx in cultured fibroblasts is decreased by the calcium-channel antagonist nifedipine or by some bivalent inorganic cations. Biochem J. 1993 Nov 1;295(Pt 3):773–779. doi: 10.1042/bj2950773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou J. C., Phelps P. C., Shin M. L., Smith M. W., Trump B. F. Effects of Ca2+ deregulation on mitochondrial membrane potential and cell viability in nucleated cells following lytic complement attack. Cell Calcium. 1994 Mar;15(3):217–227. doi: 10.1016/0143-4160(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Papadimitriou J. C., Ramm L. E., Drachenberg C. B., Trump B. F., Shin M. L. Quantitative analysis of adenine nucleotides during the prelytic phase of cell death mediated by C5b-9. J Immunol. 1991 Jul 1;147(1):212–217. [PubMed] [Google Scholar]
- Patel A. K., Campbell A. K. The membrane attack complex of complement induces permeability changes via thresholds in individual cells. Immunology. 1987 Jan;60(1):135–140. [PMC free article] [PubMed] [Google Scholar]
- Pecker F., Lotersztajn S. The liver plasma membrane Ca-ATPase. Ann N Y Acad Sci. 1982;402:438–439. doi: 10.1111/j.1749-6632.1982.tb25761.x. [DOI] [PubMed] [Google Scholar]
- Repke K. R. A model for allosteric regulation of Na+/K+-transporting ATPase. Biochim Biophys Acta. 1986 Sep 22;864(2):195–212. doi: 10.1016/0304-4157(86)90011-0. [DOI] [PubMed] [Google Scholar]
- Sala-Newby G. B., Campbell A. K. Engineering a bioluminescent indicator for cyclic AMP-dependent protein kinase. Biochem J. 1991 Nov 1;279(Pt 3):727–732. doi: 10.1042/bj2790727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolding N. J., Houston W. A., Morgan B. P., Campbell A. K., Compston D. A. Reversible injury of cultured rat oligodendrocytes by complement. Immunology. 1989 Aug;67(4):441–446. [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Page M., Campbell A. K., Luzio J. P. A mechanism for the insertion of complement component C9 into target membranes. Mol Immunol. 1986 May;23(5):451–458. doi: 10.1016/0161-5890(86)90108-2. [DOI] [PubMed] [Google Scholar]
- Stein J. M., Luzio J. P. Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem J. 1991 Mar 1;274(Pt 2):381–386. doi: 10.1042/bj2740381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. M., Luzio J. P., Campbell A. K. A method for in vitro synthesis of unglycosylated recombinant complement component C9. J Immunol Methods. 1994 Jan 3;167(1-2):129–137. doi: 10.1016/0022-1759(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Taylor K. M., Trimby A. R., Campbell A. K. Mutation of recombinant complement component C9 reveals the significance of the N-terminal region for polymerization. Immunology. 1997 May;91(1):20–27. doi: 10.1046/j.1365-2567.1997.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]