Abstract
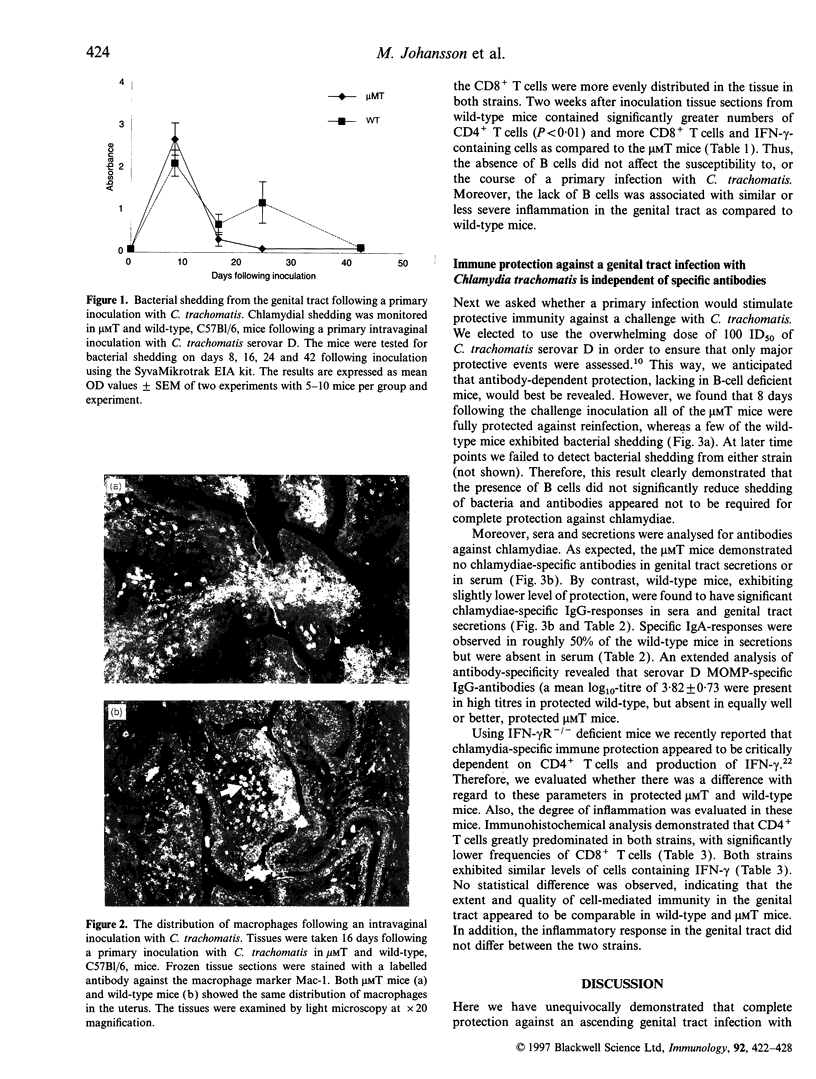
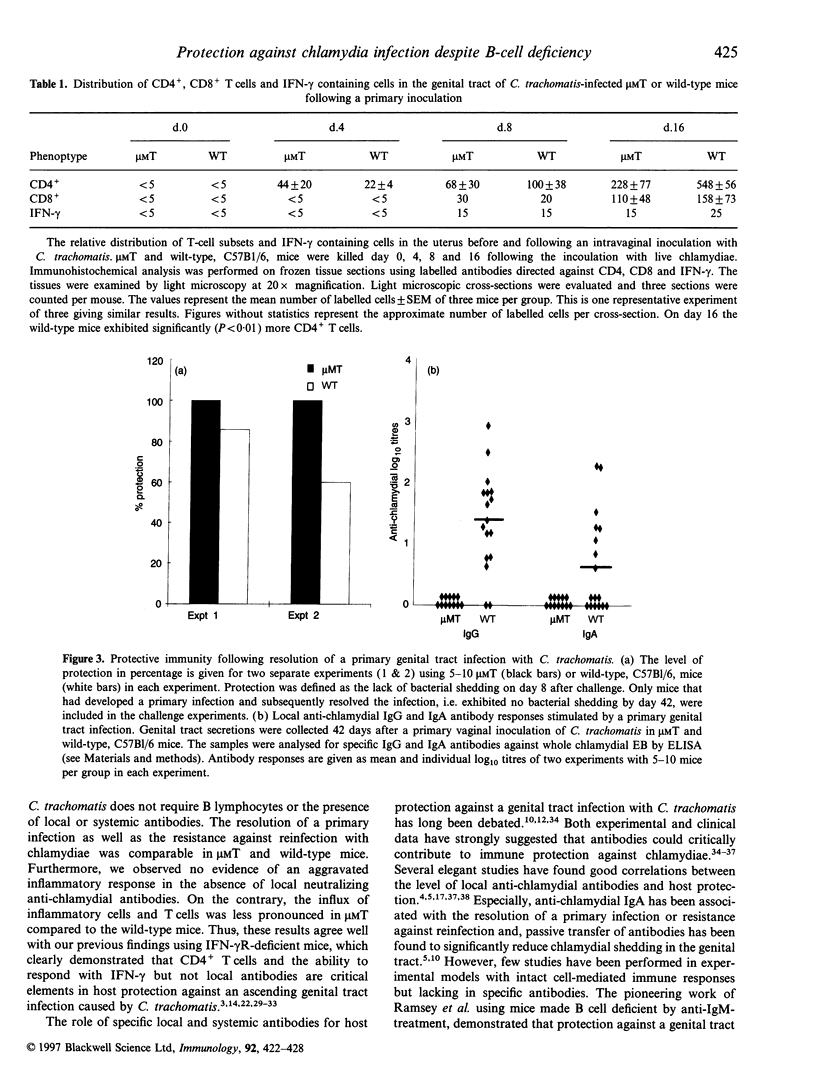
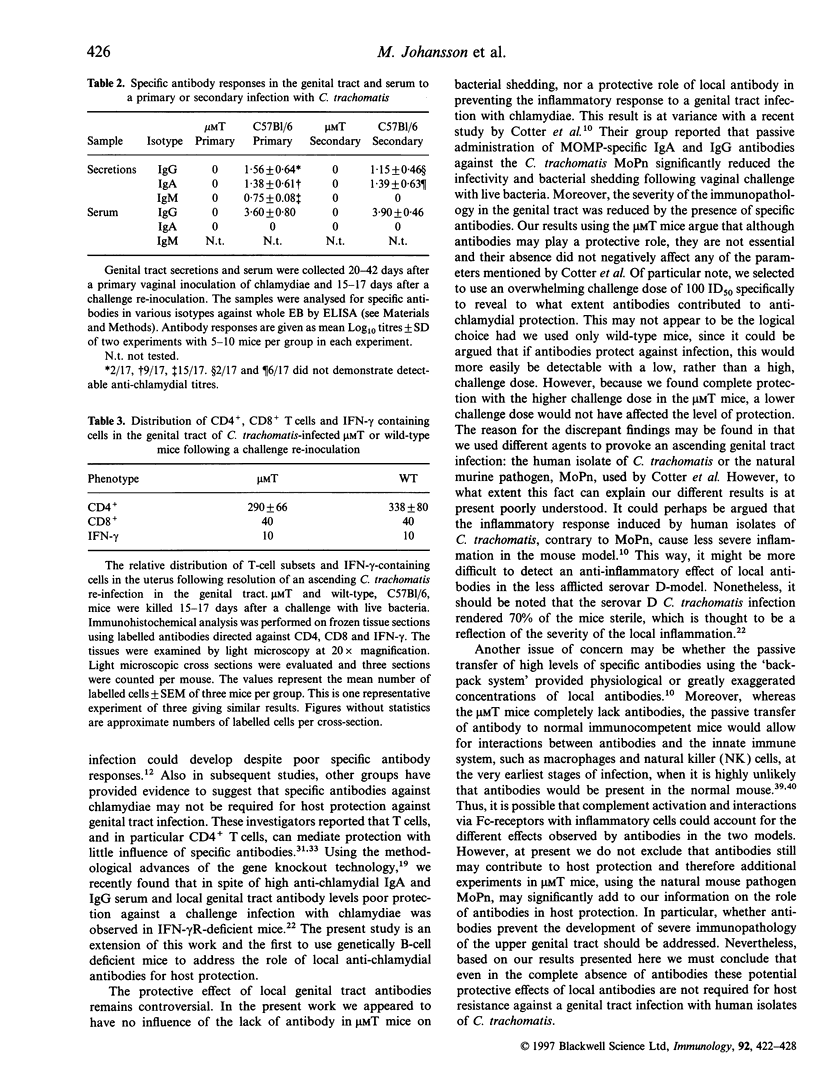
We evaluated the ability of mice made genetically deficient for B cells to resolve a primary infection and to develop protective immunity against vaginal challenge with a human isolate of Chlamydia trachomatis bacteria. The B-cell-deficient microMT mice cleared a primary ascending infection with similar or faster kinetics compared with wild-type mice. The presence of chlamydial inclusion bodies and the degree of inflammation in the upper genital tract was comparable and showed similar kinetics in microMT as in wild-type mice. Following resolution of the primary infection the mice were challenged by 100 ID50 of live bacteria and the level of protection and the extent of local inflammation was assessed. Strikingly, all microMT mice, as well as most of the wild-type mice, demonstrated complete immune protection with no bacterial shedding. While high titres of chlamydia-specific antibodies were stimulated locally and systemically in wild-type mice, no antibodies were detected in microMT mice. However, in both strains, immunohistochemical analysis of the upper genital tract demonstrated the presence of large numbers of CD4+ T cells and increased levels of interferon-gamma (IFN-gamma)-producing cells. The results unequivocally demonstrate that antibodies are not required for full protection to develop against ascending infection with a high dose of C. trachomatis in the female genital tract. Our study confirms the notion that cell-mediated immunity, in particular that owing to CD4+ T helper I (Th1)-type cells, is critical for host resistance against C. trachomatis in mice.
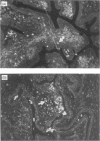
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. E., Locksley R. M., Stephens R. S. A single peptide from the major outer membrane protein of Chlamydia trachomatis elicits T cell help for the production of antibodies to protective determinants. J Immunol. 1991 Jul 15;147(2):674–679. [PubMed] [Google Scholar]
- Andersson J., Abrams J., Björk L., Funa K., Litton M., Agren K., Andersson U. Concomitant in vivo production of 19 different cytokines in human tonsils. Immunology. 1994 Sep;83(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Bailey R. L., Holland M. J., Whittle H. C., Mabey D. C. Subjects recovering from human ocular chlamydial infection have enhanced lymphoproliferative responses to chlamydial antigens compared with those of persistently diseased controls. Infect Immun. 1995 Feb;63(2):389–392. doi: 10.1128/iai.63.2.389-392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993 Aug;5(4):503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- Brunham R. C., Kuo C. C., Cles L., Holmes K. K. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect Immun. 1983 Mar;39(3):1491–1494. doi: 10.1128/iai.39.3.1491-1494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain T. K., Rank R. G. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1995 May;63(5):1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter T. W., Meng Q., Shen Z. L., Zhang Y. X., Su H., Caldwell H. D. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1995 Dec;63(12):4704–4714. doi: 10.1128/iai.63.12.4704-4714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z. D., Tristram D., LaScolea L. J., Kwiatkowski T., Jr, Kopti S., Ogra P. L. Induction of antibody response to Chlamydia trachomatis in the genital tract by oral immunization. Infect Immun. 1991 Apr;59(4):1465–1469. doi: 10.1128/iai.59.4.1465-1469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland M. J., Bailey R. L., Hayes L. J., Whittle H. C., Mabey D. C. Conjunctival scarring in trachoma is associated with depressed cell-mediated immune responses to chlamydial antigens. J Infect Dis. 1993 Dec;168(6):1528–1531. doi: 10.1093/infdis/168.6.1528. [DOI] [PubMed] [Google Scholar]
- Igietseme J. U., Rank R. G. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. Infect Immun. 1991 Apr;59(4):1346–1351. doi: 10.1128/iai.59.4.1346-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M., Schön K., Ward M., Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997 Mar;65(3):1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Bacterial and protozoal infections in genetically disrupted mice. Curr Opin Immunol. 1994 Aug;6(4):518–525. doi: 10.1016/0952-7915(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Kihlström E., Lindgren R., Rydén G. Antibodies to Chlamydia trachomatis in women with infertility, pelvic inflammatory disease and ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol. 1990 May-Jun;35(2-3):199–204. doi: 10.1016/0028-2243(90)90162-t. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Iqball S., Woods C., Stagg A., Ward M. E., Tuffrey M. A peptide of Chlamydia trachomatis shown to be a primary T-cell epitope in vitro induces cell-mediated immunity in vivo. Immunology. 1995 May;85(1):8–15. [PMC free article] [PubMed] [Google Scholar]
- Magee D. M., Williams D. M., Smith J. G., Bleicker C. A., Grubbs B. G., Schachter J., Rank R. G. Role of CD8 T cells in primary Chlamydia infection. Infect Immun. 1995 Feb;63(2):516–521. doi: 10.1128/iai.63.2.516-521.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Feilzer K., Tumas D. B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995 Dec;63(12):4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdin A. D., Su H., Klein M. H., Caldwell H. D. Poliovirus hybrids expressing neutralization epitopes from variable domains I and IV of the major outer membrane protein of Chlamydia trachomatis elicit broadly cross-reactive C. trachomatis-neutralizing antibodies. Infect Immun. 1995 Mar;63(3):1116–1121. doi: 10.1128/iai.63.3.1116-1121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry L. L., Feilzer K., Caldwell H. D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997 Apr 1;158(7):3344–3352. [PubMed] [Google Scholar]
- Pickett M. A., Ward M. E., Clarke I. N. High-level expression and epitope localization of the major outer membrane protein of Chlamydia trachomatis serovar L1. Mol Microbiol. 1988 Sep;2(5):681–685. doi: 10.1111/j.1365-2958.1988.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Ramsey K. H., Rank R. G. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect Immun. 1991 Mar;59(3):925–931. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K. H., Soderberg L. S., Rank R. G. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988 May;56(5):1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Barron A. L. Humoral immune response in acquired immunity to chlamydial genital infection of female guinea pigs. Infect Immun. 1983 Jan;39(1):463–465. doi: 10.1128/iai.39.1.463-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Batteiger B. E. Protective role of serum antibody in immunity to chlamydial genital infection. Infect Immun. 1989 Jan;57(1):299–301. doi: 10.1128/iai.57.1.299-301.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Ramsey K. H., Pack E. A., Williams D. M. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992 Oct;60(10):4427–4429. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Soderberg L. S., Sanders M. M., Batteiger B. E. Role of cell-mediated immunity in the resolution of secondary chlamydial genital infection in guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1989 Mar;57(3):706–710. doi: 10.1128/iai.57.3.706-710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., White H. J., Barron A. L. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1979 Nov;26(2):573–579. doi: 10.1128/iai.26.2.573-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. N., Ward M. E., Conway D., Caul E. O. Chlamydial and gonococcal antibodies in sera of infertile women with tubal obstruction. J Clin Pathol. 1987 Apr;40(4):377–383. doi: 10.1136/jcp.40.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Elkins C., Wyrick P. B., Cohen M. S. Vaccines for bacterial sexually transmitted infections: a realistic goal? Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2456–2463. doi: 10.1073/pnas.91.7.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Caldwell H. D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995 Sep;63(9):3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Caldwell H. D. Immunogenicity of a chimeric peptide corresponding to T helper and B cell epitopes of the Chlamydia trachomatis major outer membrane protein. J Exp Med. 1992 Jan 1;175(1):227–235. doi: 10.1084/jem.175.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Morrison R. P., Watkins N. G., Caldwell H. D. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990 Jul 1;172(1):203–212. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Parnell M., Caldwell H. D. Protective efficacy of a parenterally administered MOMP-derived synthetic oligopeptide vaccine in a murine model of Chlamydia trachomatis genital tract infection: serum neutralizing IgG antibodies do not protect against chlamydial genital tract infection. Vaccine. 1995 Aug;13(11):1023–1032. doi: 10.1016/0264-410x(95)00017-u. [DOI] [PubMed] [Google Scholar]
- Tuffrey M., Alexander F., Inman C., Ward M. E. Correlation of infertility with altered tubal morphology and function in mice with salpingitis induced by a human genital-tract isolate of Chlamydia trachomatis. J Reprod Fertil. 1990 Jan;88(1):295–305. doi: 10.1530/jrf.0.0880295. [DOI] [PubMed] [Google Scholar]
- Van der Pol B., Williams J. A., Jones R. B. Rapid antigen detection assay for identification of Chlamydia trachomatis infection. J Clin Microbiol. 1995 Jul;33(7):1920–1921. doi: 10.1128/jcm.33.7.1920-1921.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E. The immunobiology and immunopathology of chlamydial infections. APMIS. 1995 Nov;103(11):769–796. doi: 10.1111/j.1699-0463.1995.tb01436.x. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Grubbs B. Role of natural killer cells in infection with the mouse pneumonitis agent (murine Chlamydia trachomatis). Infect Immun. 1987 Jan;55(1):223–226. doi: 10.1128/iai.55.1.223-226.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]