Abstract
The nuclear hormone receptors hepatocyte nuclear factor 4 (HNF4) and the retinoid X α (RXRα) plus the peroxisome proliferator-activated receptor α (PPARα) heterodimer support hepatitis B virus (HBV) replication in nonhepatoma cells. Hepatocyte nuclear factor 3 (HNF3) inhibits nuclear hormone receptor-mediated viral replication. Inhibition of HBV replication by HNF3β is associated with the preferential reduction in the level of the pregenomic RNA compared with that of precore RNA. Hepatitis B e antigen (HBeAg), encoded by the precore RNA, mediates part of the inhibition of viral replication by HNF3β. The amino-terminal transcriptional activation domain of HNF3β is essential for the inhibition of HBV replication. The activation of transcription by HNF3 from HBV promoters downstream from the nucleocapsid promoter appears to contribute indirectly to the reduction in the steady-state level of 3.5-kb HBV RNA, possibly by interfering with the elongation rate of these transcripts. Therefore, transcriptional interference mediated by HNF3 may also regulate HBV RNA synthesis and viral replication.
Hepatitis B virus (HBV) replicates essentially exclusively in the liver by reverse transcription of the 3.5-kb pregenomic RNA, generating the partially double-stranded 3.2-kb HBV DNA genome (12, 28, 45). Viral tropism is probably determined in part by a liver-specific receptor that is required for HBV infection of hepatocytes. However, viral replication is also restricted to hepatocytes due to the essential requirement for the liver-enriched transcription factors hepatocyte nuclear factor 4 (HNF4) and the retinoid X receptor α (RXRα) plus the peroxisome proliferator-activated receptor α (PPARα) heterodimer (RXRα-PPARα) that regulate the synthesis of the pregenomic RNA (44). The level of synthesis of the pregenomic RNA is governed by the activity of the nucleocapsid promoter (15, 44). A variety of ubiquitous and liver-enriched transcription factors in addition to the nuclear hormone receptors HNF4 and RXRα-PPARα, appear to modulate the rate of transcription initiation from the nucleocapsid promoter (4, 17, 20, 22, 25, 26, 35, 43, 46, 48). However, it has been demonstrated that nuclear hormone receptor-dependent viral replication can be inhibited by the liver-enriched transcription factor HNF3 due to the preferential inhibition of pregenomic RNA synthesis relative to precore RNA synthesis (44). The mechanism of regulation of 3.5-kb HBV RNA synthesis and inhibition of viral replication by HNF3 have not been defined and are examined in the current analysis.
In this study, the functional domain of HNF3β responsible for regulating nuclear hormone receptor-dependent 3.5-kb HBV RNA synthesis and viral replication has been investigated in mouse fibroblasts. HNF3β is a member of the hepatocyte nuclear factor 3/forkhead transcription factor family (19, 21, 23). The members of this family of transcription factors are characterized by a conserved winged helix DNA binding domain that is approximately 100 amino acids in length (8, 19, 21). The HNF3 polypeptides have additional conserved sequences in the amino- and carboxyl-terminal regions, flanking the DNA binding domain that is located in the middle of the polypeptide (19). In the case of HNF3β, these conserved amino acid sequences have been shown to comprise part of the transcriptional activation domains of this polypeptide (32, 33). In this analysis, the amino-terminal transcriptional activation domain of HNF3β (32, 33) was shown to be primarily responsible for inhibiting viral replication, whereas the carboxyl-terminal transcriptional activation domain of HNF3β (32, 33) did not greatly affect HBV DNA synthesis. The inhibitory effect of this HNF3β domain on viral replication contrasts with the observation that the amino-terminal transcriptional activation domain was responsible for the increase in reporter gene expression mediated by the HBV large surface antigen and nucleocapsid promoters. These results suggested that the lower level of the 3.5-kb HBV RNA might be due to HNF3β reducing the rate of 3.5-kb HBV RNA elongation rather than negatively regulating nucleocapsid promoter activity. This possibility was supported by the observation that HNF3β could reduce viral replication when pregenomic RNA was synthesized from the cytomegalovirus (CMV) immediate-early promoter rather than the HBV nucleocapsid promoter. In addition, the ability of HNF3β to preferentially decrease the level of the pregenomic RNA compared with precore RNA produced conditions where hepatitis B e antigen (HBeAg) also contributed to the reduction in viral biosynthesis. Therefore, it appears that HNF3β inhibits HBV replication by reducing pregenomic RNA abundance by transcriptional interference and modulating the effect of HBeAg on viral biosynthesis.
MATERIALS AND METHODS
Plasmid constructions.
The steps in the cloning of the plasmid constructs used in the transfection experiments were performed by standard techniques (38). HBV DNA sequences in these constructions were derived from the pCP10 plasmid, which contains two copies of the HBV genome (subtype ayw) cloned into the EcoRI site of pBR322 (10). The firefly luciferase (LUC) reporter gene in these constructions was derived from the p19DLUC plasmid (36). The CpLUC plasmid contains one complete HBV genome located directly 5′ to the promoterless LUC reporter gene such that the expression of the LUC gene is governed by the HBV nucleocapsid promoter (36). Similarly, the PS1pLUC plasmid contains one complete HBV genome located directly 5′ to the promoterless LUC reporter gene such that the expression of the LUC gene is governed by the HBV large surface antigen promoter (36). Details of the construction of the CpLUC and PS1pLUC plasmids have been described previously (36, 47).
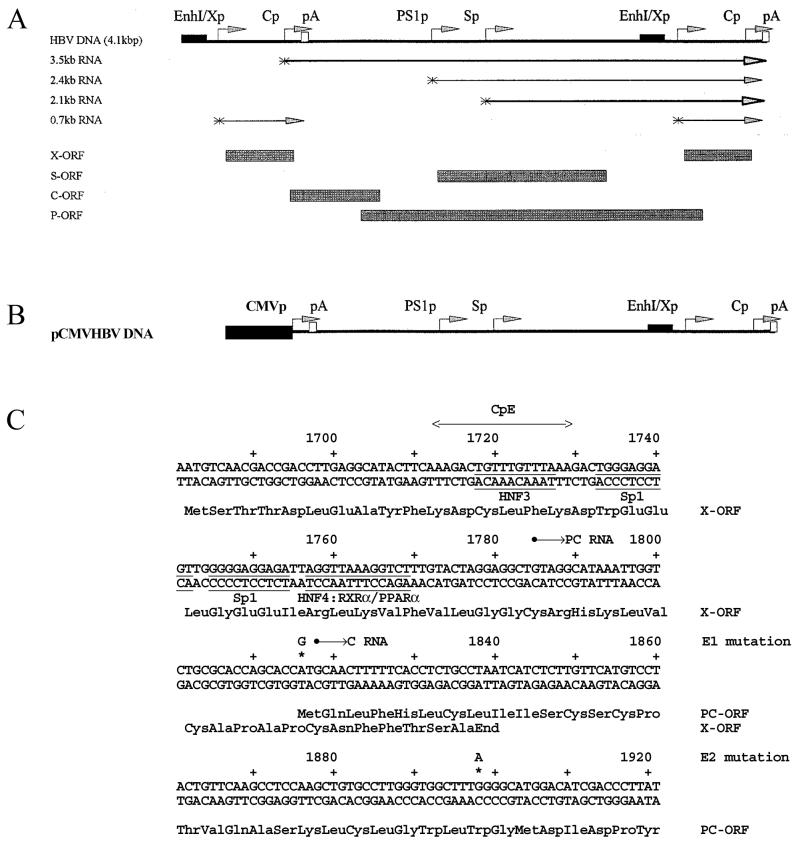
The HBV DNA (4.1-kbp) construct that contains 1.3 copies of the HBV genome includes the viral sequence from nucleotide coordinates 1072 to 3182 plus 1 to 1990 (Fig. 1A). This plasmid was constructed by cloning the NsiI/BglII HBV DNA fragment (nucleotide coordinates 1072 to 1990) into pUC13, generating pHBV(1072-1990). Subsequently, a complete copy of the 3.2-kbp viral genome linearized at the NcoI site (nucleotide coordinates 1375 to 3182 plus 1 to 1374) was cloned into the unique NcoI site (HBV nucleotide coordinate 1374) of pHBV(1072-1990), generating the HBV DNA (4.1-kbp) construct. The pCMVHBVayw construct contains the CMV immediate-early promoter (region from nucleotide coordinates −522 to −1) (3) located directly upstream of the HBV sequence from nucleotide coordinates 1821 to 3182 plus 1 to 1990 (Fig. 1B). In this construct, the expression of the HBV pregenomic 3.5-kb RNA is controlled by the CMV immediate-early promoter.
FIG. 1.
Structures and sequences of the HBV constructs supporting viral transcription and replication in mouse fibroblasts. (A) Structure of the HBV DNA (4.1-kbp) construct used in transient-transfection analysis. The 4.1-kbp greater-than-genome length HBV DNA sequence in this construct spans coordinates 1072 to 3182 plus 1 to 1990 of the HBV genome (subtype ayw). The locations of the 3.5-, 2.4-, 2.1-, and 0.7-kb HBV transcripts are indicated. EnhI/Xp, enhancer I/X-gene promoter region; Cp, nucleocapsid or core promoter; pA, polyadenylation site; PS1p, presurface antigen promoter; Sp, major surface antigen promoter; X, X gene; S, surface antigen gene; C, core gene; P, polymerase gene; ORF, open reading frame. (B) Structure of the pCMVHBV DNA construct used in transient-transfection analysis. The CMV immediate-early promoter (region from coordinates −522 to −1) directs the expression of the 3.5-kb HBV pregenomic RNA from the greater-than-genome length HBV DNA sequence in this construct that spans coordinates 1821 to 3182 plus 1 to 1990 of the HBV genome (subtype ayw). The locations of the 3.5-, 2.4-, 2.1-, and 0.7-kb HBV transcripts are the same as indicated for the HBV DNA (4.1-kbp) construct. (C) Sequence of the HBV core promoter region. The E1 (A1816G) and E2 (G1898A) mutations in the precore open reading frame (PC-ORF) prevent the expression of HBeAg from the HBV DNA (4.1-kbp) E1 and E2 mutant constructs. The sequence of the X-gene-encoded polypeptide is not changed by the E1 mutation in the X-gene open reading frame (X-ORF). The location of the CpE double-stranded oligonucleotide (HNF3 recognition site) used for electrophoretic mobility shift analysis is indicated. The HNF3, Sp1, and nuclear hormone receptor (HNF4 and RXRα-PPARα) binding sites are also indicated. PC RNA, precore 3.5-kb RNA; C RNA, pregenomic 3.5-kb RNA.
The HBV DNA (4.1-kbp) E1mut and E2mut constructs were derived by introducing the A1816G and G1898A nucleotide substitutions (Fig. 1C), respectively, into the precore coding region in the HBV DNA (4.1-kbp) construct using the Chameleon double-stranded, site-directed mutagenesis kit (Stratagene Cloning Systems, La Jolla, Calif.) according to the manufacturer's instructions. The A1816G nucleotide substitution converted the precore initiation codon from ATG to GTG, preventing the initiation of translation of the precore polypeptide. The G1898A nucleotide substitution converted the precore polypeptide TGG codon 28 encoding tryptophan to the amber translation termination TAG codon, resulting in the premature termination of the precore polypeptide (1, 18, 31). The nucleotide substitutions introduced into the precore coding regions were verified by dideoxynucleotide sequencing (39). Both precore coding regions in these terminally redundant HBV constructs were mutated for this analysis.
The pMTHNF1α, pMTHNF1β, pCMVHNF3α, pCMVHNF3β, pCMVHNF4, pRS-hRXRα, and pCMVPPARα-G vectors express HNF1α, HNF1β, HNF3α, HNF3β, HNF4, RXRα, and PPARα-G polypeptides from the rat HNF1α, mouse HNF1β, rat HNF3α, rat HNF3β, rat HNF4, human RXRα, and mouse PPARα-G cDNAs, respectively, using the mouse metallothionein I promoter, the CMV immediate-early promoter (pCMV), or the Rous sarcoma virus long terminal repeat (pRS) (6, 27, 30, 34, 35, 37). The PPARα-G polypeptide contains a mutation in the PPARα cDNA changing Glu282 to Gly that may decrease the affinity of the receptor for the endogenous ligand. Consequently, this mutation increases the peroxisome proliferator-dependent (i.e., clofibric acid-dependent) activation of transcription from a peroxisome proliferator response element (PPRE) containing promoter (30) and was used in this study to demonstrate the peroxisome proliferator-dependent transcriptional transactivation of the nucleocapsid promoter.
Cells and transfections.
The mouse NIH 3T3 fibroblast cell line was grown in RPMI 1640 medium and 10% fetal bovine serum at 37°C in 5% CO2 and air. Transfections using luciferase reporter gene constructs were performed as previously described (14, 41), except six-well plates, containing approximately 3 × 105 cells per well, were used. The transfected DNA mixture comprised 5 μg of a LUC plasmid and 0.25 μg of pCMVβ, which served as an internal control for transfection efficiency. pCMVβ directs the expression of the Escherichia coli β-galactosidase gene using the CMV immediate-early promoter (Clontech Laboratories, Palo Alto, Calif.). When appropriate, the DNA mixture also included 0.5 μg of the HNF3β expression vectors pCMVHNF3β, pCMVHNF3β1-444, pCMVHNF3β1-392, pCMVHNF3β1-366, pCMVHNF3β1-309, pCMVHNF3β103-458, pCMVHNF3β153-458, and pCMVHNF3β144-279 or the control expression vector pCMV. The numbers at the end of the plasmid designation indicate the amino acid residues present in the truncated HNF3β polypeptides encoded by these expression vectors (32, 33). The DNA was removed 4 to 6 h after transfection, and the cells were washed with 2 ml of fresh RPMI 1640 medium. Cell extracts were prepared 40 to 48 h after transfection. Cells were lysed in 150 μl of lysis buffer [0.1 M potassium phosphate (pH 7.8), 0.2% (vol/vol) Triton X-100], and the cell debris was pelleted by centrifugation for 2 min at 13,000 rpm in an Eppendorf 5417C microcentrifuge. The supernatant was assayed for luciferase activity essentially as previously described (9) and for β-galactosidase activity using a Galacto-Light kit (Tropix, Inc.) as instructed by the manufacturer. The level of β-galactosidase activity observed was not specifically affected by any of the exogenously expressed transcription factors. The luciferase activities were normalized to the level of β-galactosidase activity in each transfection experiment.
Transfections for viral RNA and DNA analyses were performed as previously described (29) using 10-cm-diameter plates, containing approximately 106 cells. DNA and RNA isolation was performed 3 days posttransfection. The transfected DNA mixture was composed of 10 μg of HBV DNA (4.1 kbp) plus 1.5 μg of the liver-enriched transcription factor expression vectors pMTHNF1α, pMTHNF1β, pCMVHNF3α, pCMVHNF3β, pCMVHNF4, pRS-hRXRα, and pCMVPPARα-G (6, 27, 30, 34, 35, 37, 44). Controls were derived from cells transfected with HBV DNA and the pCMV expression vector lacking a liver-enriched transcription factor cDNA insert (35). All-trans retinoic acid and clofibric acid at 1 μM and 1 mM, respectively, were used to activate the nuclear hormone receptors RXRα and PPARα (44).
Characterization of HBV transcripts and viral replication intermediates.
Transfected cells from a single plate were divided equally and used for the preparation of total cellular RNA and viral DNA replication intermediates as described previously (42) with minor modifications. For RNA isolation (7), the cells were lysed in 1.8 ml of a solution containing 25 mM sodium citrate (pH 7.0), 4 M guanidinium isothiocyanate, 0.5% (vol/vol) sarcosyl, and 0.1 M 2-mercaptoethanol. After addition of 0.18 ml of 2 M sodium acetate (pH 4.0), the lysate was extracted with 1.8 ml of water-saturated phenol plus 0.36 ml of chloroform-isoamyl alcohol (49:1). After centrifugation for 30 min at 3,000 rpm in a Sorval RT6000 centrifuge, the aqueous layer was precipitated with 1.8 ml of isopropanol. The precipitate was resuspended in a solution containing 0.3 ml of 25 mM sodium citrate (pH 7.0), 4 M guanidinium isothiocyanate, 0.5% (vol/vol) sarcosyl, and 0.1 M 2-mercaptoethanol and precipitated with 0.6 ml of ethanol. After centrifugation for 20 min at 14,000 rpm in an Eppendorf 5417C microcentifuge, the precipitate was resuspended in 0.3 ml of a solution containing 10 mM Tris hydrochloride (pH 8.0), 5 mM EDTA, and 0.1% (wt/vol) sodium lauryl sulfate and precipitated with 45 μl of 2 M sodium acetate plus 0.7 ml of ethanol.
For the isolation of viral DNA replication intermediates, the cells were lysed in 0.4 ml of 100 mM Tris hydrochloride (pH 8.0) plus 0.2% (vol/vol) Nonidet P-40. The lysate was centrifuged for 1 min at 14,000 rpm in an Eppendorf 5417C microcentrifuge to pellet the nuclei. The supernatant was adjusted to 6.75 mM magnesium acetate plus 200 μg of DNase I per ml and incubated for 1 h at 37°C to remove the transfected plasmid DNA. The supernatant was readjusted to 100 mM NaCl, 10 mM EDTA, 0.8% (wt/vol) sodium lauryl sulfate, and 1.6 mg of pronase per ml and incubated for an additional 1 h at 37°C. The supernatant was extracted twice with phenol, precipitated with 2 volumes of ethanol and resuspended in 100 μl of 10 mM Tris hydrochloride (pH 8.0) plus 1 mM EDTA. RNA (Northern) and DNA (Southern) filter hybridization analyses were performed using 10 μg of total cellular RNA and 30 μl of viral DNA replication intermediates, respectively, as described previously (38).
RNase protection assays were performed using the Pharmingen Riboquant kit, and riboprobes were synthesized using the Ambion Maxiscript kit as described by the manufacturers. Transcription initiation sites for the 3.5-kb HBV transcripts were examined using 20 μg of total cellular RNA and a 333-nucleotide-long (HBV coordinates 1990 to 1658) 32P-labeled HBV riboprobe. As an internal control for the RNase protection analysis, a 32P-labeled mouse ribosomal protein L32 gene riboprobe spanning 101 nucleotides of exon 3 was utilized (11). All riboprobes contained additional flanking vector sequences of 40 to 90 nucleotides that are not protected by HBV RNA.
Whole-cell extracts and gel retardation analysis.
Whole-cell extracts were prepared from mouse NIH 3T3 fibroblasts by a rapid micropreparation technique as described previously (2). Mouse fibroblasts were transfected with 15 μg of the expression vectors encoding the truncated HNF3β polypeptides 40 to 48 h before preparation of the whole-cell extracts. Gel retardation analysis was performed essentially as described previously (34, 37). One nanogram of 32P-labeled CpE double-stranded oligonucleotide was incubated with 4 μl of whole-cell extract prior to 4% polyacrylamide gel electrophoresis and autoradiography. The CpE double-stranded oligonucleotides spanning a HNF3 site in the nucleocapsid promoter have been described previously (20).
RESULTS
Expression of truncated HNF3β polypeptides in mouse fibroblasts.
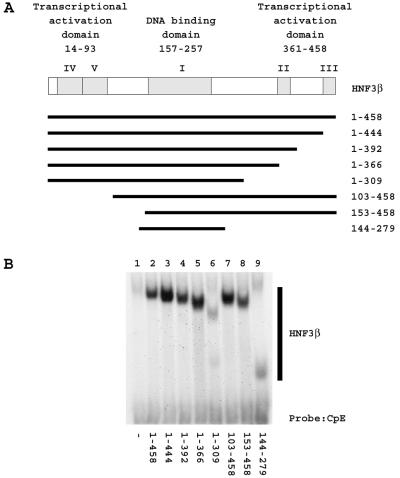
HNF3 has previously been shown to activate transcription from the HBV nucleocapsid promoter in reporter gene analysis (37) and to inhibit pregenomic RNA synthesis and viral biosynthesis in HBV replication analysis (44). In an attempt to examine these apparently contradictory observations, the functional domains of HNF3β modulating HBV transcription and replication were examined. If the same domain of HNF3 were found to activate transcription from the HBV nucleocapsid promoter in reporter gene analysis and to inhibit pregenomic RNA synthesis and viral biosynthesis in HBV replication analysis, the same mechanism may be involved in both processes. In contrast, if different domains of HNF3 were found to be involved in these processes, different mechanisms may also be involved. Initially, various truncated HNF3β polypeptides lacking either the amino- or carboxyl-terminal transcriptional activation domains (32, 33) were expressed in mouse NIH 3T3 fibroblasts (Fig. 2A). Electrophoretic mobility shift analysis demonstrated that the truncated HNF3β polypeptides were expressed at similar levels and retained the ability to bind to a HNF3 recognition sequence present in the HBV nucleocapsid promoter (Fig. 2B). The truncated HNF3β polypeptide spanning amino acids 1 to 309 (Fig. 2B, lane 6) displayed the lowest level of DNA binding activity but was completely functional with respect to modulating HBV transcription and replication (Fig. 3 and 4). Therefore, it appears that any differences in the level of expression of the truncated HNF3β polypeptides used in this analysis did not influence greatly the function of these transcription factors.
FIG. 2.
Expression of truncated HNF3β polypeptides in mouse NIH 3T3 fibroblasts. (A) Schematic representations of the HNF3β polypeptides showing the locations of the transcriptional activation domains and the DNA binding domain (32, 33) The amino-terminal transcriptional activation domain includes conserved sequence regions IV and V (33). The carboxyl-terminal transcriptional activation domain includes conserved sequence regions II and III (32). The winged helix DNA binding domain spans the conserved sequence region I (8, 32, 33). The amino acids of the truncated HNF3β polypeptides are shown to the right of the schematic representations. (B) Electrophoretic mobility shift analysis of a HBV nucleocapsid promoter HNF3 recognition site with truncated HNF3β polypeptides. The 32P-labeled, double-stranded oligonucleotide CpE (20) and whole-cell extracts prepared from mouse fibroblasts transfected with an empty vector control (−) (lane 1), an expression vector encoding the full-length HNF3β polypeptide (amino acid residues 1 to 458) (lane 2), and expression vectors encoding the truncated HNF3β polypeptides spanning amino acid residues 1 to 444 (lane 3), 1 to 392 (lane 4), 1 to 366 (lane 5), 1 to 309 (lane 6), 103 to 458 (lane 7), 153 to 458 (lane 8), and 144 to 279 (lane 9) were used for this analysis.
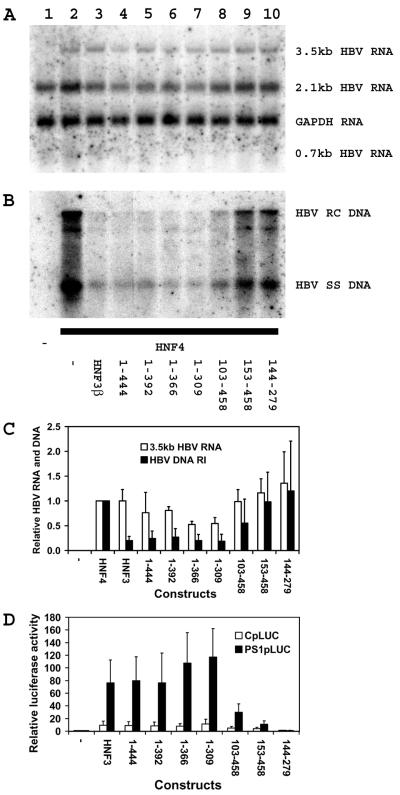
FIG. 3.
Modulation of HNF4-dependent HBV transcription and replication by truncated HNF3β polypeptides. Mouse NIH 3T3 fibroblasts were transiently transfected with the HBV DNA (4.1-kbp) construct (lanes 1 to 10) plus the HNF4 expression vector (lanes 2 to 10) and the HNF3β expression vectors (lanes 3 to 10). (A) RNA (Northern) filter hybridization analysis of HBV transcripts. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcript was used as an internal control for RNA loading per lane. (B) DNA (Southern) filter hybridization analysis of HBV replication intermediates. HBV RC DNA, HBV relaxed circular DNA; HBV SS DNA, HBV single-stranded DNA. The amino acids of the HNF3β polypeptides for panels A and B are shown below the gel in panel B. (C) Quantitative analysis of the 3.5-kb HBV RNA and DNA replication intermediates. The levels of the 3.5-kb HBV RNA and HBV DNA replication intermediates (HBV DNA RI) are reported relative to the levels of the HBV DNA (4.1-kbp) construct in the presence of HNF4 expression (lane 2), which are set at 1.0. The mean RNA and DNA levels plus standard deviations (indicated by the error bars) from two independent analyses are shown. (D) The effects of truncated HNF3β polypeptides on transcription from the nucleocapsid and large surface antigen promoter constructs CpLUC and PS1pLUC, respectively, were examined. Relative activities of the constructs in mouse fibroblast in the absence orpresence of ectopically expressed truncated HNF3β polypeptides are indicated. The amino acids of the HNF3β polypeptides are indicated below the graph. The transcriptional activities are reported relative to those of the CpLUC and PS1pLUC constructs in the absence of HNF3β expression (−), with a relative activity set at 1.0. The internal control used to correct for transfection efficiencies was pCMVβ. The mean luciferase activities plus standard deviations (indicated by the error bars) from three independent analyses are shown.
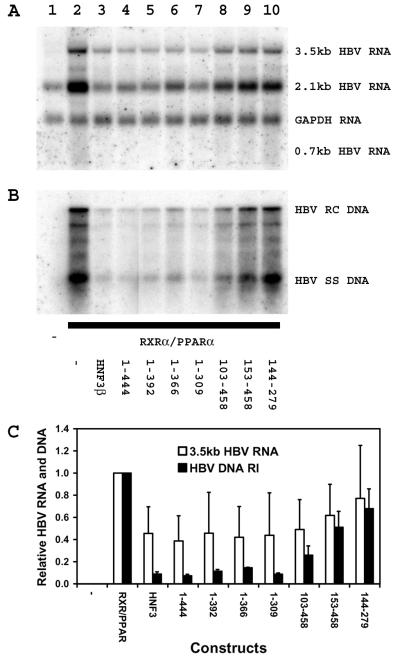
FIG. 4.
Modulation of RXRα-PPARα-dependent HBV transcription and replication by truncated HNF3β polypeptides. Mouse NIH 3T3 fibroblasts were transiently transfected with the HBV DNA (4.1-kbp) construct (lanes 1 to 10) plus the RXRα-PPARα expression vector (lanes 2 to 10) and the HNF3β expression vectors (lanes 3 to 10). (A) RNA (Northern) filter hybridization analysis of HBV transcripts. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcript was used as an internal control for RNA loading per lane. (B) DNA (Southern) filter hybridization analysis of HBV replication intermediates. HBV RC DNA, HBV relaxed circular DNA; HBV SS DNA, HBV single-stranded DNA. The amino acids of the HNF3β polypeptides in panels A and B are shown below the gel in panel B. (C) Quantitative analysis of the 3.5-kb HBV RNA and HBV DNA replication intermediates. The levels of the 3.5-kb HBV RNA and HBV DNA replication intermediates (HBV DNA RI) are reported relative to those of the HBV DNA (4.1-kbp) construct in the presence of RXRα-PPARα expression (lane 2), which are set at 1.0. All-trans retinoic acid and clofibric acid at 1 μM and 1 mM, respectively, were used to activate the nuclear hormone receptors RXRα and PPARα. The mean RNA and DNA levels plus standard deviations (indicated by the error bars) from two independent analyses are shown. The amino acids of the HNF3β polypeptides are shown below the graph.
Identification of the HNF3β polypeptide domains involved in regulating HBV transcription and replication in mouse fibroblasts.
The domain of the HNF3β polypeptide involved in inhibiting HNF4-mediated HBV transcription and replication was examined in mouse NIH 3T3 fibroblasts (Fig. 3A to C). It is apparent that the carboxyl-terminal domain of the HNF3β polypeptide was not required for the inhibition of viral transcription and replication. In contrast, inhibition of viral transcription and replication was reduced when the amino-terminal domain of the HNF3β polypeptide was deleted (Fig. 3A to C, lanes 9). This indicates that the amino-terminal transcriptional activation domain located in the first 153 amino acids of HNF3β was necessary to inhibit HBV transcription and replication. The truncated HNF3β polypeptide spanning amino acids 103 to 458 (Fig. 3A to C, lanes 8) appeared to partially inhibit HBV RNA and DNA syntheses. This observation suggested that the functional domain of HNF3β modulating HBV transcription and replication may include polypeptide sequences located between amino acid residues 1 and 102 in addition to amino acid residues between coordinates 103 and 152. The DNA binding domain of HNF3β located between amino acid residues 144 and 279 was unable to inhibit HNF4-mediated HBV transcription and replication (Fig. 3A to C) despite retaining the ability to bind to a HNF3 recognition element (Fig. 2B). This is consistent with the mapping of the HNF3β domain inhibiting HBV transcription and replication within the amino-terminal region of this transcription factor. In addition, it is apparent that the full-length and carboxyl-terminus-truncated HNF3β polypeptides inhibited viral replication to a greater extent than HBV transcription. These observations suggested that these HNF3β polypeptides might decrease the level of synthesis of the pregenomic RNA to a greater extent than that of the precore RNA (44).
Despite the ability of HNF3β to inhibit HNF4-mediated HBV transcription and replication, HNF3β can activate transcription from the nucleocapsid promoter in the context of a reporter gene construct in mouse NIH 3T3 fibroblasts (Fig. 3D). Therefore, the domain of the HNF3β polypeptide responsible for activating transcription in this context was examined using the HBV nucleocapsid and large surface antigen promoter constructs CpLUC and PS1pLUC, respectively. HNF3β activates transcription from these promoters approximately 10- and 75-fold, respectively. It is apparent that the carboxyl-terminal domain of the HNF3β polypeptide was not required for the activation of transcription from the nucleocapsid or large surface antigen promoters. In contrast, the truncated polypeptides lacking the amino-terminal transcriptional activation domain located in the first 153 amino acids of HNF3β displayed a greatly reduced ability to increase the level of transcription from either the nucleocapsid or large surface antigen promoter (Fig. 3D). As observed for the inhibition of HBV transcription and replication, deletion of the first 102 amino acids of HNF3β resulted in a partial loss of transcriptional activation (Fig. 3D, compare HNF3β and HNF3β103-458). Transcriptional activation was reduced further from the HBV promoters when the complete amino-terminal domain was deleted from the HNF3β polypeptide (Fig. 3D, compare HNF3β and HNF3β153-458). Therefore, it is apparent that the amino-terminal transcriptional activation domain of HNF3β mediated the majority of both the activation of transcription from the nucleocapsid and large surface antigen promoters using the reporter gene constructs and the inhibition of viral transcription and replication from the HBV DNA (4.1-kbp) construct in mouse fibroblasts. In addition, it appears that the extent to which the amino-terminal deletions activated transcription correlates with the degree to which HBV transcription and replication are inhibited. The observation that HNF3β activates transcription from HBV reporter gene constructs under the same conditions where HBV RNA and DNA synthesis are inhibited eliminates the possibility that HNF3β inhibits viral transcription and replication by the process of squelching (13).
The amino-terminal transcriptional activation domain of HNF3β was also predominantly responsible for the inhibition of RXRα-PPARα-mediated HBV transcription and replication in mouse fibroblasts (Fig. 4). This indicates that HNF3β can inhibit HBV transcription and replication that is dependent on more than a single nuclear hormone receptor and suggests that HNF3β may indirectly influence HBV pregenomic RNA synthesis in the context of viral replication. As observed with HNF4, the amino-terminal region between residues 1 and 102 partially inhibited HBV transcription and replication (Fig. 4A and B, lanes 8). However, deletion of the complete amino-terminal domain of HNF3β (Fig. 4A and B, lanes 9) essentially eliminated the capacity of this polypeptide to modulate HBV RNA and DNA syntheses. These observations support the suggestion that amino acid sequences within the first 102 residues and between residues 103 to 152 contribute to the functional domain of HNF3β responsible for the inhibition of HBV transcription and replication.
Role of HBeAg in the inhibition of HBV replication by HNF3β in mouse fibroblasts.
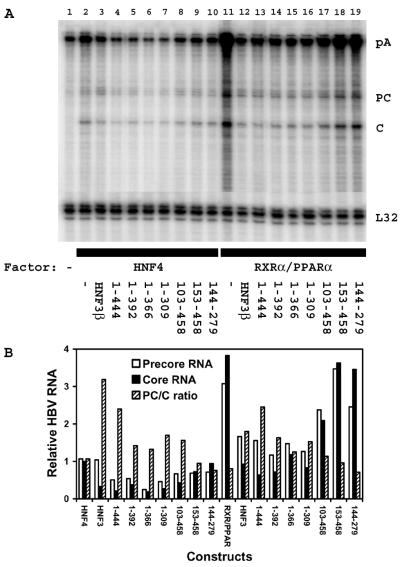
It was previously observed that HNF3 inhibition of nuclear hormone receptor-mediated HBV replication in mouse NIH 3T3 fibroblasts was associated with a greater decrease in the pregenomic RNA than in the precore RNA (44). This results in a modest increase in the precore RNA/pregenomic RNA ratio (Fig. 5). The effect of the truncated HNF3β polypeptides on the relative abundance of the precore and pregenomic RNAs was examined (Fig. 5). It appears that the truncated HNF3β polypeptides lacking the carboxyl-terminal transcriptional activation domain also inhibited the synthesis of the pregenomic RNA to a greater extent than that of the precore RNA (Fig. 5, lanes 4 to 7 and 13 to 16) although possibly to a lesser extent than the full-length HNF3β polypeptide (Fig. 5, lanes 3 and 12). In contrast, the relative abundance of the precore and pregenomic RNAs observed in the absence of HNF3β (Fig. 5, lanes 2 and 11) was similar to that observed in mouse fibroblasts transfected with the truncated HNF3β polypeptides lacking the complete amino-terminal transcriptional activation domain (Fig. 5, lanes 9 and 10 and lanes 18 and 19). Therefore, it appears that HNF3β polypeptides that efficiently inhibit HBV replication also preferentially decrease the level of pregenomic RNA relative to the precore RNA (Fig. 5B). Under these circumstances, it appears that the reduction in the level of viral replication is somewhat greater than the reduction in pregenomic RNA synthesis (Fig. 3 to 5). This indirect evidence tentatively suggested that the translation product of the precore RNA, the HBeAg polypeptide, might contribute to the inhibition of viral replication. This possibility is supported by previous observations suggesting that HBeAg can inhibit HBV replication (5, 16, 40).
FIG. 5.
Effects of truncated HNF3β polypeptides on the relative levels of precore and pregenomic RNA synthesis. Mouse NIH 3T3 fibroblasts were transiently transfected with the HBV DNA (4.1-kbp) construct (lanes 1 to 19), the HNF4 expression vector (lanes 2 to 10), the RXRα-PPARα expression vectors (RXRα/PPARα) (lanes 11 to 19), and the truncated HNF3β expression vectors (lanes 3 to 10 and 12 to 19) as indicated. The amino acids of the truncated HNF3β expression vectors are shown below the gel. (A) RNase protection analysis was performed to map the transcription initiation sites of the HBV precore (PC) and pregenomic or core (C) transcripts. The HBV probe also protected a fragment (pA) derived from the 3′ ends of all the HBV RNAs that terminated at the HBV polyadenylation site. A riboprobe detecting the ribosomal gene L32 transcripts was included as an internal control. (B) Quantitative analysis of the 3.5-kb HBV precore (PC) and core (C) RNA levels. The levels of the 3.5-kb HBV PC and C RNAs are reported relative to those of the C RNA transcribed from the HBV DNA (4.1-kbp) construct in the presence of HNF4 expression (lane 2), which are set at 1.0. The quantitative analyses of lanes 2 to 19 in panel A are shown.
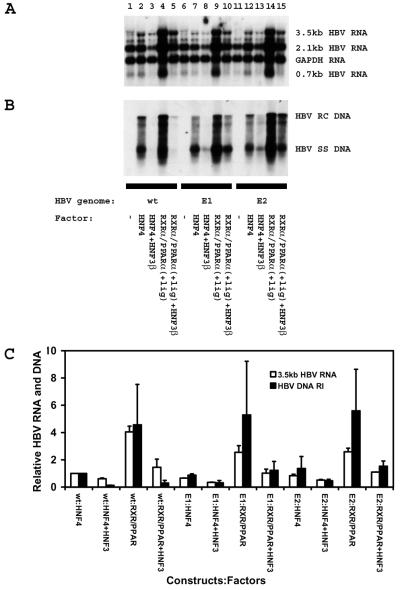
The possibility that HBeAg expression might contribute to the inhibition of viral replication by HNF3β in mouse fibroblasts was investigated directly (Fig. 6). Two replication-competent viral constructs, HBV DNA (4.1-kbp) E1mut and E2mut, were examined to determine if HNF3β can inhibit viral replication in the absence of HBeAg synthesis. It was observed that HBeAg synthesis does not appear to inhibit HNF4-mediated (Fig. 6A, lanes 2, 7, and 12) or RXRα-PPARα-mediated viral replication (Fig. 6A, lanes 4, 9, and 14). However, it is apparent that HNF3β inhibits HNF4- and RXRα-PPARα-mediated wild-type viral replication approximately 8- and 16-fold, respectively (Fig. 6A, lanes 2 to 5). In contrast, HNF3β inhibits HNF4- and RXRα-PPARα-mediated viral replication approximately three- and fourfold, respectively, from the HBV DNA (4.1-kbp) E1mut construct that cannot express HBeAg (Fig. 6A, lanes 7 to 10). HNF3β also inhibits HNF4- and RXRα-PPARα-mediated viral replication approximately three- and fourfold, respectively, from the HBV DNA (4.1-kbp) E2mut construct that also cannot express HBeAg (Fig. 6A, lanes 12 to 15). In contrast to the different effects of HNF3β on viral replication, HNF3β reduced 3.5-kb RNA synthesis from the wild-type and HBeAg-minus viral genomes to similar extents (Fig. 6). Therefore, it is appears that HNF3β inhibits viral replication from the wild-type viral genome partly by an HBeAg-dependent mechanism. This presumably occurs because HNF3β decreases the level of the HBeAg-encoding precore RNA less than the core polypeptide-encoding pregenomic RNA. Consequently, it appears that HBeAg can inhibit viral replication efficiently only when the pregenomic RNA level is low relative to the precore RNA, as HBeAg does not appear to affect viral replication in the absence of HNF3β (Fig. 6A, lanes 2, 4, 7, 9, 12, and 14). However, HNF3β can inhibit viral replication from constructs that cannot express HBeAg. This indicates that HNF3β also inhibits viral replication by directly reducing the level of pregenomic RNA in addition to altering the relative level of HBeAg expression (Fig. 6).
FIG. 6.
Transcription and replication of wild-type and HBeAg-minus HBV DNA (4.1-kbp) constructs in mouse NIH 3T3 fibroblasts. Cells were transiently transfected with the wild-type (wt) HBV DNA (4.1-kbp) construct (lanes 1 to 5) and the HBeAg-minus HBV DNA (4.1-kbp) constructs (E1 [lanes 6 to 10] and E2 [lanes 11 to 15]) and liver-enriched transcription factors as indicated. (A) RNA (Northern) filter hybridization analysis of HBV transcripts. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcript was used as an internal control for RNA loading per lane. (B) DNA (Southern) filter hybridization analysis of HBV replication intermediates. HBV RC DNA, HBV relaxed circular DNA; HBV SS DNA, HBV single-stranded DNA. All-trans retinoic acid and clofibric acid at 1 μM and 1 mM, respectively, were used to activate the nuclear hormone receptors RXRα and PPARα. (C) Quantitative analysis of the 3.5-kb HBV RNA and HBV DNA replication intermediates. The levels of the 3.5-kb HBV RNA and HBV DNA replication intermediates (HBV DNA RI) are reported relative to those of the HBV DNA (4.1-kbp) construct in the presence of HNF4 expression (lane 2), which are set at 1.0. The mean RNA and DNA levels plus standard deviations (indicated by the error bars) from two independent analyses are shown.
HNF3β inhibits HBV pregenomic RNA synthesis from a heterologous promoter in mouse fibroblasts.
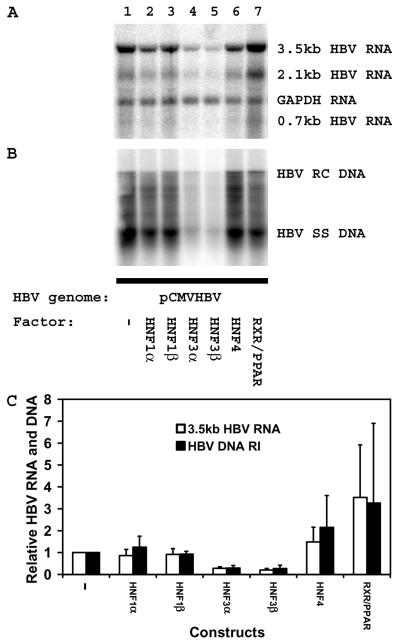
Nuclear hormone receptor-mediated HBV pregenomic RNA synthesis regulated by the nucleocapsid promoter is inhibited by HNF3β in mouse NIH 3T3 fibroblasts (Fig. 5 and 6). In contrast, HNF3β activates transcription from the nucleocapsid promoter when the transcript encodes the luciferase gene (Fig. 3D). This suggests that HBV regulatory elements present within the transcribed region of the HBV DNA (4.1-kbp) construct might be involved in inhibiting the synthesis of the pregenomic RNA. The observations that HNF3 recognition sequences are present in all the HBV promoters and transcription of the pregenomic RNA requires that RNA synthesis transverses these regulatory regions suggest HNF3β might inhibit pregenomic RNA synthesis by interfering with pregenomic RNA elongation. If this were the case, the nature of the promoter directing the expression of the pregenomic RNA should not influence the ability of HNF3β to inhibit pregenomic RNA synthesis and HBV replication. The pCMVHBVayw construct directs the expression of the pregenomic RNA from the CMV immediate-early promoter. Viral replication occurs from the pregenomic RNA synthesized from this construct in the absence of nuclear hormone receptors in mouse fibroblasts (Fig. 7A and B, lanes 1). The ectopic expression of HNF1α, HNF1β, HNF4, and RXRα-PPARα does not inhibit viral transcription or replication (Fig. 7, lanes 2, 3, 6, and 7). HNF4 and RXRα-PPARα appear to modestly enhance viral transcription and replication from the pCMVHBVayw construct. In contrast, HNF3α and HNF3β inhibit viral transcription and replication approximately fourfold. HNF3 does not directly affect the activity of the CMV immediate-early promoter (H. Tang and A. McLachlan, unpublished data). These observations suggest that HNF3 may inhibit pregenomic RNA synthesis from a regulatory sequence element, probably a HNF3 recognition sequence, located downstream from the transcription initiation site of the pregenomic RNA. This appears to represent a new level of transcriptional regulation that may ensure that transcription from the HBV promoters is coordinately regulated so that appropriate levels of viral products are synthesized to support efficient viral biosynthesis.
FIG. 7.
HNF3β inhibits HBV pregenomic RNA synthesis and viral replication from regulatory elements downstream from the nucleocapsid promoter in the mouse NIH 3T3 fibroblasts. Cells were transiently transfected with the pCMVHBV DNA construct (lanes 1 to 7) and liver-enriched transcription factors (lanes 2 to 7) as indicated. (A) RNA (Northern) filter hybridization analysis of HBV transcripts. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcript was used as an internal control for RNA loading per lane. (B) DNA (Southern) filter hybridization analysis of HBV replication intermediates. HBV RC DNA, HBV relaxed circular DNA; HBV SS DNA, HBV single-stranded DNA. All-trans retinoic acid and clofibric acid at 1 μM and 1 mM, respectively, were used to activate the nuclear hormone receptors RXRα and PPARα. (C) Quantitative analysis of the 3.5-kb HBV RNA and DNA replication intermediates. The levels of the 3.5-kb HBV RNA and HBV DNA replication intermediates (HBV DNA RI) are reported relative to those of the pCMVHBV DNA construct in the absence of ectopic transcription factor expression (lane 1), which are set at 1.0. The mean RNA and DNA levels plus standard deviations (indicated by the error bars) from three independent analyses are shown.
DISCUSSION
HBV replication is restricted to hepatocytes partly because pregenomic RNA synthesis is regulated by liver-enriched transcription factors (44). Transcriptional regulation of HBV by the nuclear hormone receptors HNF4 and RXRα plus PPARα is a critical determinant in restricting viral replication to the liver (44). However, additional liver-enriched transcription factors can modulate nuclear hormone receptor-mediated HBV transcription and replication in mouse fibroblasts. HNF3 negatively regulates viral transcription and replication in this system (44). This observation was unexpected, as HNF3 has been demonstrated to activate transcription from the nucleocapsid promoter in reporter gene analysis (20, 24, 37). Therefore, it appeared that in the context of viral replication, HNF3 was affecting viral 3.5-kb RNA transcription from HBV regulatory elements located within the transcribed region of pregenomic RNA.
The mechanism of action of HNF3β on HBV transcription and replication was investigated in mouse fibroblasts using both viral replication and reporter gene analyses. In both cases, it was shown that the amino-terminal transcriptional activation domain of HNF3β (32, 33) was responsible for inhibiting HBV replication in the viral replication analysis and for activating transcription from the nucleocapsid promoter in the reporter gene analysis (Fig. 3 and 4). These results suggested the inhibition of viral replication is mediated by the activation of transcription from one or more of the HBV promoters.
Previously, it had been suggested that HNF3β preferentially inhibited the transcription of the pregenomic RNA compared with that of precore RNA (44). This observation was confirmed and shown to be dependent on the presence of the amino-terminal transcriptional activation domain in the HNF3β polypeptide (Fig. 5). This suggested that HNF3β influences the selection of the transcription initiation site from the nucleocapsid promoter, and consequently, the level of the HBeAg-encoding precore RNA is reduced less than the core polypeptide-encoding pregenomic RNA. The fact that the decrease in viral replication due to HNF3β expression is larger than expected for the reduction in pregenomic RNA suggested that the higher relative level of HBeAg synthesis compared with that of the core polypeptide might contribute to the HNF3β-mediate inhibition of viral replication. HBeAg has been shown to inhibit HBV replication in a variety of systems (5, 16, 40). This possibility was investigated by examining the ability of HNF3β to inhibit viral replication from mutated HBV genomic DNA templates that were not capable of expressing HBeAg (Fig. 6). Under these circumstances, HNF3β inhibited HBV replication but to a considerably lesser extent than from a template that encoded the HBeAg polypeptide. This analysis indicated that HBeAg contributes to the inhibition of viral replication in mouse fibroblasts under certain circumstances. HNF3β mediates the inhibition of viral replication partly by decreasing the level of precore RNA less than that of the pregenomic RNA and consequently decreasing the level of HBeAg less than that of the core polypeptide. If the HBeAg polypeptide can inhibit replication-competent capsid assembly from occurring as has been suggested (40), a greater decrease in core polypeptide synthesis than that in HBeAg synthesis could result in a larger than expected decrease in viral biosynthesis. However, alterations in the relative levels of the precore and pregenomic RNAs can account for only part of the HNF3β-mediated inhibition of nuclear hormone receptor-mediated viral replication.
It is apparent that HNF3β directly inhibits the level of the pregenomic RNA. This effect is not dependent of the nature of the promoter that is directing the expression of the pregenomic RNA (Fig. 7). When the CMV immediate-early promoter is used to direct the expression of the HBV pregenomic RNA, HNF3α and HNF3β reduced both the levels of this transcript and viral replication. This demonstrated that HNF3 inhibits HBV transcription and replication from regulatory elements located within the pregenomic RNA transcription unit. As the amino-terminal transcriptional activation domain is required to inhibit pregenomic RNA synthesis and viral replication, these observations suggest that HNF3β inhibits pregenomic RNA synthesis by promoting transcription from a downstream HBV promoter such as the large surface antigen, major surface antigen, or the X-gene promoters. As the 2.1-kb HBV RNA level is reduced by HNF3β expression (Fig. 3, 4, 6 and 7), it is unlikely that the reduction in 3.5-kb HBV RNA synthesis results from HNF3-mediated transcription from the major surface antigen promoter. However, it suggests that the formation of a transcriptionally active preinitiation complex containing HNF3 at one of the other HBV promoters downstream from the nucleocapsid promoter may restrict the efficiency of elongation of the pregenomic RNA through this region of the viral genome. This would reduce the level of the pregenomic RNA and inhibit viral replication. This is regulation of viral replication by transcriptional interference and represents an additional novel level of regulation of HBV replication.
Although less direct, the possibility that HNF3 might activate the transcription of cellular gene(s) that may selectively increase the rate of turnover of the HBV transcripts cannot be excluded. This possibility would also result in a reduction in pregenomic RNA abundance and the inhibition of viral replication. Additional studies will be required to define the detailed steps mediated by HNF3 that control the steady-state level of the HBV pregenomic RNA. A role for the X-gene product in the inhibition of viral replication by HNF3 can be excluded, as viral genomes that do not encode this polypeptide are susceptible to HNF3-mediated inhibition of HBV DNA synthesis (Tang and McLachlan, unpublished).
Acknowledgments
We are grateful to Amy Brideau and Stefan Wieland (The Scripps Research Institute, La Jolla, Calif.) for plasmid pCMVHBVayw; Eric F. Johnson (The Scripps Research Institute) for plasmids pCMVHNF4 and pCMVPPARα-G; Ronald M. Evans (The Salk Institute, La Jolla, Calif.) for plasmid pRS-hRXRα; Robert Costa (University of Illinois, Chicago, Ill.) for plasmids pCMVHNF3α, pCMVHNF3β, pCMVHNF3β1-444, pCMVHNF3β1-392, pCMVHNF3β1-366, pCMVHNF3β1-309, pCMVHNF3β103-458, pCMVHNF3β153-458, and pCMVHNF3β144-279; Riccardo Cortese (Instituto di Ricerche di Biologia Molecolare, Rome, Italy) for plasmid pB1.1 (rat HNF1α cDNA); and Gerald R. Crabtree (Stanford University, Stanford, Calif.) for plasmid 28-1 (mouse HNF1β cDNA).
This work was supported by a postdoctoral fellowship from the West China University of Medical Sciences of the People's Republic of China to H.T. and by Public Health Service grant AI30070 from the National Institutes of Health.
Footnotes
Publication number 14563-CB from The Scripps Research Institute, La Jolla, Calif.
REFERENCES
- 1.Akahane, Y., T. Yamanaka, H. Suzuki, Y. Sugai, F. Tsuda, S. Yotsumoto, S. Omi, H. Okamoto, Y. Miyakawa, and M. Mayumi. 1990. Chronic active hepatitis with hepatitis B virus DNA and antibody against e antigen in the serum. Disturbed synthesis and secretion of e antigen from hepatocytes due to a point mutation in the precore region. Gastroenterology 99:1113-1119. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, N. C., and D. V. Faller. 1991. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 19:2499.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boshart, M., F. Weber, G. Jahn, K. Dorsch-Hasler, B. Fleckenstein, and W. Schaffner. 1985. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell 41:521-530. [DOI] [PubMed] [Google Scholar]
- 4.Buckwold, V. E., M. Chen, and J. H. Ou. 1997. Interaction of transcription factors RFX1 and MIBP1 with the gamma motif of the negative regulatory element of the hepatitis B virus core promoter. Virology 227:515-518. [DOI] [PubMed] [Google Scholar]
- 5.Buckwold, V. E., Z. C. Xu, M. Chen, T. S. B. Yen, and J. H. Ou. 1996. Effects of a naturally occurring mutation in the hepatitis B virus basal core promoter on precore gene expression and viral replication. J. Virol. 70:5845-5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, D., G. Lepar, and B. Kemper. 1994. A transcriptional regulatory element common to a large family of hepatic cytochrome P450 genes is a functional binding site of the orphan receptor HNF-4. J. Biol. Chem. 269:5420-5427. [PubMed] [Google Scholar]
- 7.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 8.Clark, K. L., E. D. Halay, E. Lai, and S. K. Burley. 1993. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature 364:412-420. [DOI] [PubMed] [Google Scholar]
- 9.De Wet, J. R., K. V. Wood, M. DeLuca, D. R. Helinski, and S. Subramani. 1987. Firefly luciferase gene: structure and expression in mammalian cells. Mol. Cell. Biol. 7:725-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois, M. F., C. Pourcel, S. Rousset, C. Chany, and P. Tiollais. 1980. Excretion of hepatitis B surface antigen particles from mouse cells transformed with cloned viral DNA. Proc. Natl. Acad. Sci. USA 77:4549-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudov, K. P., and R. P. Perry. 1984. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell 37:457-468. [DOI] [PubMed] [Google Scholar]
- 12.Ganem, D., and H. E. Varmus. 1987. The molecular biology of the hepatitis B viruses. Annu. Rev. Biochem. 56:651-693. [DOI] [PubMed] [Google Scholar]
- 13.Gill, G., and M. Ptashne. 1988. Negative effect of the transcriptional activator GAL4. Nature 334:721-724. [DOI] [PubMed] [Google Scholar]
- 14.Graham, F. L., and A. J. Van der Eb. 1973. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52:456-467. [DOI] [PubMed] [Google Scholar]
- 15.Guidotti, L. G., C. M. Eggers, A. K. Raney, S. Y. Chi, J. M. Peters, F. J. Gonzalez, and A. McLachlan. 1999. In vivo regulation of hepatitis B virus replication by peroxisome proliferators. J. Virol. 73:10377-10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guidotti, L. G., B. Matzke, C. Pasquinelli, J. M. Shoenberger, C. E. Rogler, and F. V. Chisari. 1996. Hepatitis B virus (HBV) precore protein inhibits HBV replication in transgenic mice. J. Virol. 70:7056-7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo, W., M. Chen, T. S. B. Yen, and J.-H. Ou. 1993. Hepatocyte-specific expression of the hepatitis B virus core promoter depends on both positive and negative regulation. Mol. Cell. Biol. 13:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Günther, S., L. Fischer, I. Pult, M. Sterneck, and H. Will. 1999. Naturally occurring variants of hepatitis B virus. Adv. Virus Res. 52:25-137. [DOI] [PubMed] [Google Scholar]
- 19.Hromas, R., and R. Costa. 1995. The hepatocyte nuclear factor-3/forkhead transcription regulatory family in development, inflammation, and neoplasia. Crit. Rev. Oncol. Hematol. 20:129-140. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, J. L., A. K. Raney, and A. McLachlan. 1995. Characterization of a functional hepatocyte nuclear factor 3 binding site in the hepatitis B virus nucleocapsid promoter. Virology 208:147-158. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann, E., and W. Knöchel. 1996. Five years on the wings of fork head. Mech. Dev. 57:3-20. [DOI] [PubMed] [Google Scholar]
- 22.Lai, C. K., and L. P. Ting. 1999. Transcriptional repression of human hepatitis B virus genes by a bZIP family member, E4BP4. J. Virol. 73:3197-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai, E., K. L. Clark, S. K. Burley, and J. E. Darnell, Jr. 1993. Hepatocyte nuclear factor 3/fork head or “winged helix” proteins: a family of transcription factors of diverse biologic function. Proc. Natl. Acad. Sci. USA 90:10421-10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, M., Y. H. Xie, X. Wu, Y. Y. Kong, and Y. Wang. 1995. HNF3 binds and activates the second enhancer, ENII, of hepatitis B virus. Virology 214:371-378. [DOI] [PubMed] [Google Scholar]
- 25.López-Cabrera, M., J. Letovsky, K.-Q. Hu, and A. Siddiqui. 1990. Multiple liver-specific factors bind to the hepatitis B virus core/pregenomic promoter: trans-activation and repression by CCAAT/enhancer binding protein. Proc. Natl. Acad. Sci. USA 87:5069-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López-Cabrera, M., J. Letovsky, K.-Q. Hu, and A. Siddiqui. 1991. Transcriptional factor C/EBP binds to and transactivates the enhancer element II of the hepatitis B virus. Virology 183:825-829. [DOI] [PubMed] [Google Scholar]
- 27.Mangelsdorf, D. J., E. S. Ong, J. A. Dyck, and R. M. Evans. 1990. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature 345:224-229. [DOI] [PubMed] [Google Scholar]
- 28.McLachlan, A. 1991. Molecular biology of the hepatitis B virus. CRC Press, Boca Raton, Fla.
- 29.McLachlan, A., D. R. Milich, A. K. Raney, M. G. Riggs, J. L. Hughes, J. Sorge, and F. V. Chisari. 1987. Expression of hepatitis B virus surface and core antigens: influences of pre-S and precore sequences. J. Virol. 61:683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muerhoff, A. S., K. J. Griffin, and E. F. Johnson. 1992. The peroxisome proliferator-activated receptor mediates the induction of CYP4A6, a cytochrome P450 fatty acid omega-hydroxylase, by clofibric acid. J. Biol. Chem. 267:19051-19053. [PubMed] [Google Scholar]
- 31.Naoumov, N. V., R. Schneider, T. Grötzinger, M. C. Jung, S. Miska, G. R. Pape, and H. Will. 1992. Precore mutant hepatitis B virus infection and liver disease. Gastroenterology 102:538-543. [DOI] [PubMed] [Google Scholar]
- 32.Pani, L., D. G. Overdier, A. Porcella, X. Qian, E. Lai, and R. H. Costa. 1992. Hepatocyte nuclear factor 3β contains two transcriptional activation domains, one of which is novel and conserved with the Drosophila forked head protein. Mol. Cell. Biol. 12:3723-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian, X., and R. H. Costa. 1995. Analysis of hepatocyte nuclear factor-3β protein domains required for transcriptional activation and nuclear targeting. Nucleic Acids Res. 23:1184-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raney, A. K., A. J. Easton, D. R. Milich, and A. McLachlan. 1991. Promoter-specific transactivation of hepatitis B virus transcription by a glutamine- and proline-rich domain of hepatocyte nuclear factor 1. J. Virol. 65:5774-5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raney, A. K., J. L. Johnson, C. N. A. Palmer, and A. McLachlan. 1997. Members of the nuclear receptor superfamily regulate transcription from the hepatitis B virus nucleocapsid promoter. J. Virol. 71:1058-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raney, A. K., D. R. Milich, A. J. Easton, and A. McLachlan. 1990. Differentiation-specific transcriptional regulation of the hepatitis B virus large surface antigen gene in human hepatoma cell lines. J. Virol. 64:2360-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raney, A. K., P. Zhang, and A. McLachlan. 1995. Regulation of transcription from the hepatitis B virus large surface antigen promoter by hepatocyte nuclear factor 3. J. Virol. 69:3265-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scaglioni, P. P., M. Melegari, and J. R. Wands. 1997. Posttranscriptional regulation of hepatitis B virus replication by the precore protein. J. Virol. 71:345-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorge, J., D. Wright, V. D. Erdman, and A. E. Cutting. 1984. Amphotropic retrovirus vector system for human cell gene transfer. Mol. Cell. Biol. 4:1730-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Summers, J., P. M. Smith, M. Huang, and M. Yu. 1991. Morphogenetic and regulatory effects of mutations in the envelope proteins of an avian hepadnavirus. J. Virol. 65:1310-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun, C. T., W. Y. Lo, I. H. Wang, Y. H. Lo, S. R. Shiou, C. K. Lai, and L. P. Ting. 2001. Transcription repression of human hepatitis B virus genes by negative regulatory element-binding protein/SON. J. Biol. Chem. 276:24059-24067. [DOI] [PubMed] [Google Scholar]
- 44.Tang, H., and A. McLachlan. 2001. Transcriptional regulation of hepatitis B virus by nuclear hormone receptors is a critical determinant of viral tropism. Proc. Natl. Acad. Sci. USA 98:1841-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Will, H., W. Reiser, T. Weimer, E. Pfaff, M. Buscher, R. Sprengle, R. Cattaneo, and H. Schaller. 1987. Replication strategy of human hepatitis B virus. J. Virol. 61:904-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuh, C.-H., and L.-P. Ting. 1991. C/EBP-like proteins binding to the functional box-α and box-β of the second enhancer of hepatitis B virus. Mol. Cell. Biol. 11:5044-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, P., and A. McLachlan. 1994. Differentiation-specific transcriptional regulation of the hepatitis B virus nucleocapsid gene in human hepatoma cell lines. Virology 202:430-440. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, P., A. K. Raney, and A. McLachlan. 1993. Characterization of functional Sp1 transcription factor binding sites in the hepatitis B virus nucleocapsid promoter. J. Virol. 67:1472-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]