Abstract
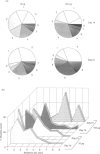
The immunization schedule is critical for the derivation of high-affinity antibodies, low antigen dose being particularly favourable for the development of a more efficient memory response. To analyse the molecular events underpinning this preference, we analysed the early maturation of the response to the hapten 2-phenyloxazolone (phOx) using low and high doses of immunogen. The phOx response is initially dominated by antibodies expressing the VkOx1-Jk5 light chain and the hallmark of the early stages of maturation is the substitution of His 34 by Asn or Gln increasing affinity 10- or eightfold, respectively, and of Tyr 36 by Phe. High-affinity antibodies express mutations at both sites. We cloned and sequenced VkOx1-Jk5 light chains from antigen-specific B cells taken 14 and 21 days after immunization with high and low antigen doses. We found that overall, the derived sequences were more mutated both at longer times and at higher dose. At day 14, His 34 was more frequently mutated at the higher than at the lower dose, while at day 21 the reverse was true. On the other hand, the His 34/Tyr 36 mutation pair was more frequent at low than high doses at both 14 and 21 days. Furthermore, at both times, the low immunization protocol yielded double mutants in cells with a lower mutation background. It appears therefore that while the higher dose may favour the acquisition of individual critical mutations, low-dose immunization favours the selection of a more focused mutational pattern, whereby advantageous mutations are associated with a low mutational background.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel M., Berek C. Somatic mutations in antibodies expressed by germinal centre B cells early after primary immunization. Int Immunol. 1990;2(9):813–819. doi: 10.1093/intimm/2.9.813. [DOI] [PubMed] [Google Scholar]
- Berek C., Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987 Apr;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Berek C., Ziegner M. The maturation of the immune response. Immunol Today. 1993 Aug;14(8):400–404. doi: 10.1016/0167-5699(93)90143-9. [DOI] [PubMed] [Google Scholar]
- Betz A. G., Neuberger M. S., Milstein C. Discriminating intrinsic and antigen-selected mutational hotspots in immunoglobulin V genes. Immunol Today. 1993 Aug;14(8):405–411. doi: 10.1016/0167-5699(93)90144-a. [DOI] [PubMed] [Google Scholar]
- Betz A. G., Rada C., Pannell R., Milstein C., Neuberger M. S. Passenger transgenes reveal intrinsic specificity of the antibody hypermutation mechanism: clustering, polarity, and specific hot spots. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2385–2388. doi: 10.1073/pnas.90.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goidl E. A., Paul W. E., Siskind G. W., Benacerraf B. The effect of antigen dose and time after immunization on the amount and affinity of anti-hapten antibody. J Immunol. 1968 Feb;100(2):371–375. [PubMed] [Google Scholar]
- González-Fernández A., Milstein C. Analysis of somatic hypermutation in mouse Peyer's patches using immunoglobulin kappa light-chain transgenes. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9862–9866. doi: 10.1073/pnas.90.21.9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984 Nov 15;312(5991):271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G., Rajewsky K., Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991 Dec 5;354(6352):389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Paul W. E., Goidl E. A., Benacerraf B. Carrier function in anti-hapten immune responses. I. Enhancement of primary and secondary anti-hapten antibody responses by carrier preimmunization. J Exp Med. 1970 Aug 1;132(2):261–282. doi: 10.1084/jem.132.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsoe G. In situ studies of the germinal center reaction. Adv Immunol. 1995;60:267–288. doi: 10.1016/s0065-2776(08)60587-8. [DOI] [PubMed] [Google Scholar]
- Kepler T. B., Perelson A. S. Cyclic re-entry of germinal center B cells and the efficiency of affinity maturation. Immunol Today. 1993 Aug;14(8):412–415. doi: 10.1016/0167-5699(93)90145-B. [DOI] [PubMed] [Google Scholar]
- Källberg E., Gray D., Leanderson T. The effect of carrier and carrier priming on the kinetics and pattern of somatic mutation in the V chi Ox1 gene. Eur J Immunol. 1995 Aug;25(8):2349–2354. doi: 10.1002/eji.1830250834. [DOI] [PubMed] [Google Scholar]
- Küppers R., Zhao M., Hansmann M. L., Rajewsky K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 1993 Dec 15;12(13):4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhout E., Mevissen M. L., Kwekkeboom J., Tager J. M., de Groot C. Direct evidence that human follicular dendritic cells (FDC) rescue germinal centre B cells from death by apoptosis. Clin Exp Immunol. 1993 Feb;91(2):330–336. doi: 10.1111/j.1365-2249.1993.tb05904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. J., Joshua D. E., Williams G. T., Smith C. A., Gordon J., MacLennan I. C. Mechanism of antigen-driven selection in germinal centres. Nature. 1989 Dec 21;342(6252):929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Rada C., Gupta S. K., Gherardi E., Milstein C. Mutation and selection during the secondary response to 2-phenyloxazolone. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5508–5512. doi: 10.1073/pnas.88.13.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokat K. M., Goodnow C. C. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995 May 25;375(6529):334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Weiss U., Zoebelein R., Rajewsky K. Accumulation of somatic mutants in the B cell compartment after primary immunization with a T cell-dependent antigen. Eur J Immunol. 1992 Feb;22(2):511–517. doi: 10.1002/eji.1830220233. [DOI] [PubMed] [Google Scholar]
- Ziegner M., Steinhauser G., Berek C. Development of antibody diversity in single germinal centers: selective expansion of high-affinity variants. Eur J Immunol. 1994 Oct;24(10):2393–2400. doi: 10.1002/eji.1830241020. [DOI] [PubMed] [Google Scholar]