Abstract
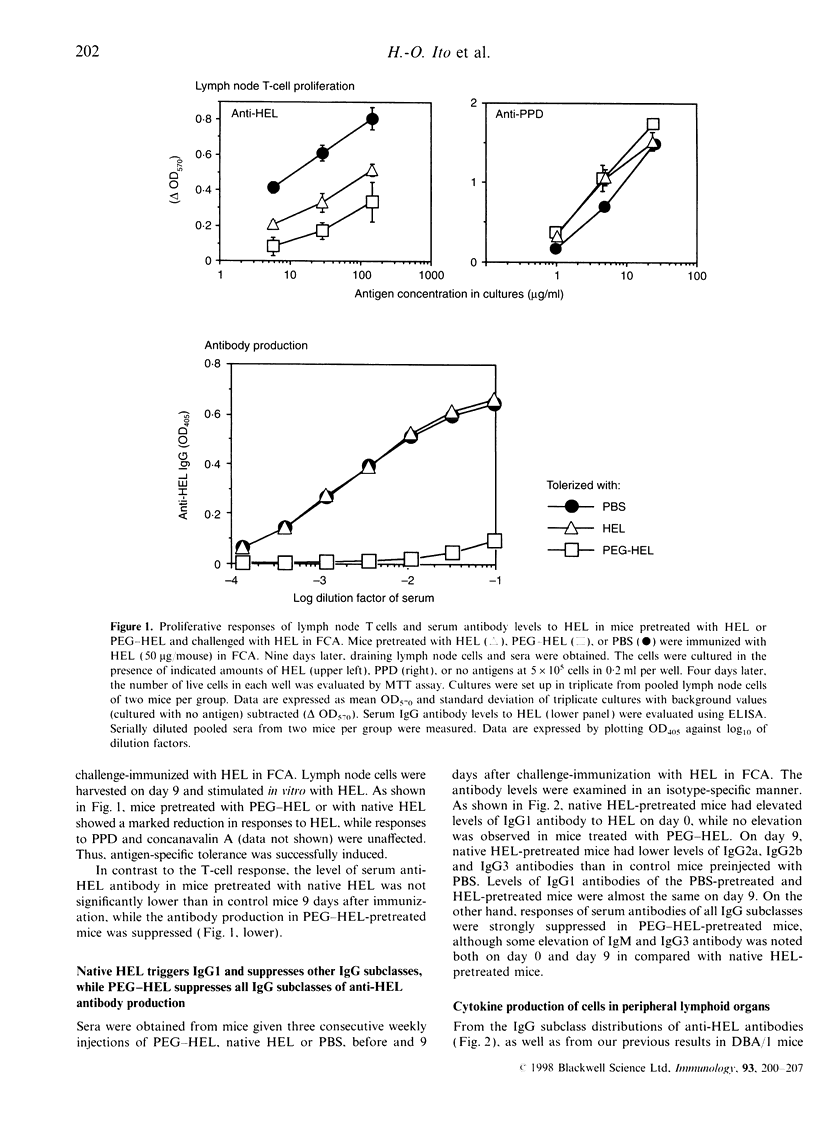
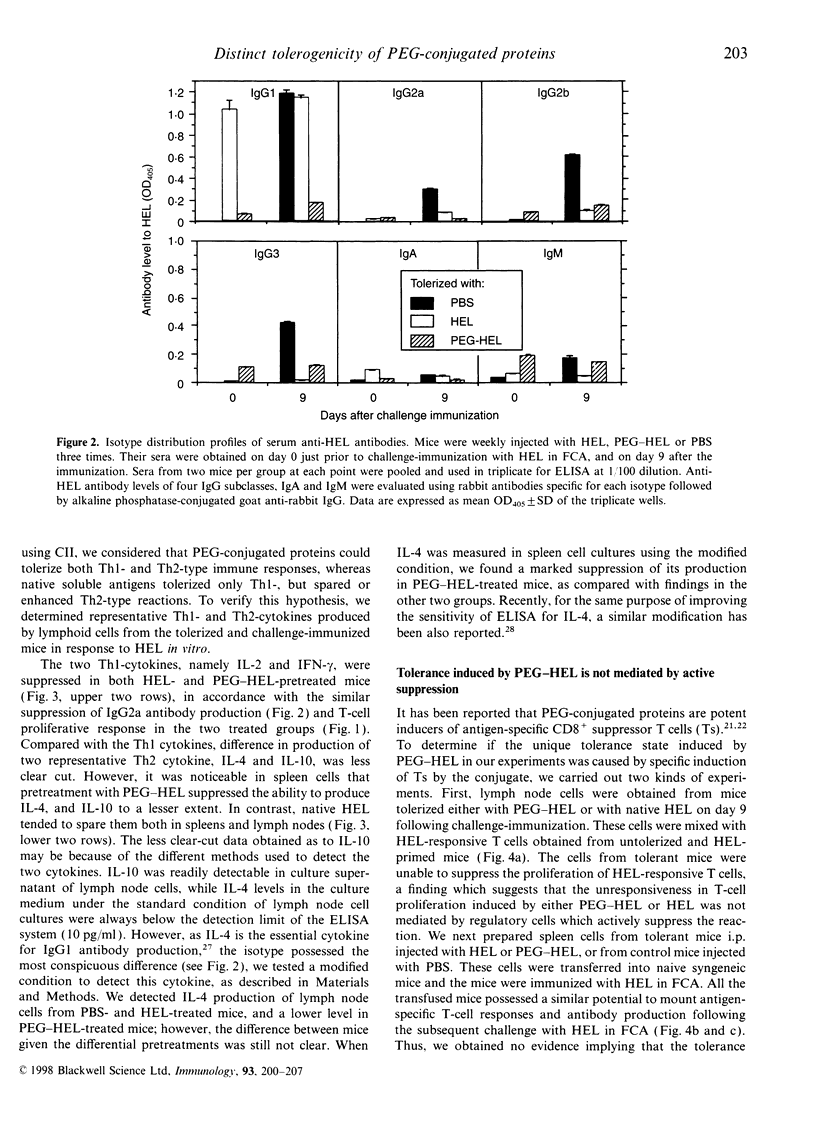
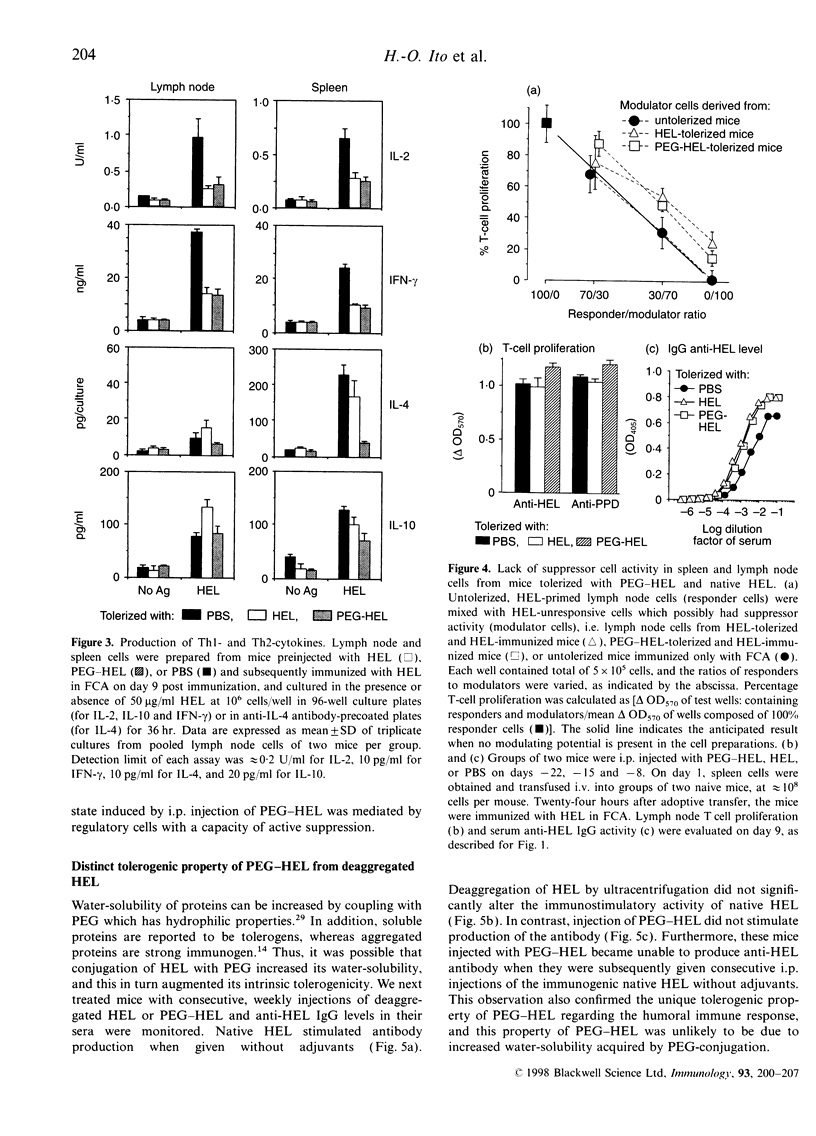
Conjugation of proteins with polyethylene glycol (PEG) has been reported to make the proteins tolerogenic. Native proteins are also potentially tolerogenic when given without adjuvants. We compared immunotolerogenic activities between PEG-conjugated and native hen egg-white lysozyme (HEL). BALB/c mice were given consecutive weekly intraperitoneal administrations of PEG-conjugated HEL, unmodified HEL or phosphate-buffered saline (PBS), for 3 weeks, then challenged with HEL in Freund's complete adjuvant. The pretreatment with PEG-HEL tolerized both T-cell and humoral responses, while native HEL tolerized only the T-cell response. Immunoglobulin G1 (IgG1) antibody was already elevated in HEL-pretreated mice prior to challenge immunization, followed by suppressed IgG2a and IgG2b, but spared IgG1 production after the antigen challenge, whereas PEG-HEL-pretreated mice produced no antibody in all IgG subclasses prior and subsequent to the antigen-challenge. Production of interleukin-2 (IL-2) and interferon-gamma (IFN-gamma) by lymphoid cells in response to HEL in vitro was markedly suppressed in both the antigen-pretreated groups, while suppression of IL-4 production was evident only in PEG-HEL-, not in HEL-pretreated animals. Involvement of suppressor cells in these tolerance states was found to be unlikely, and the immunological property of PEG-HEL differed from deaggregated HEL that was similar to the original HEL. These results suggest a unique immunotolerogenic activity of PEG-conjugated proteins to suppress both T-helper type-1 (Th1)- and Th2-type responses, the result being extensive inhibition of all IgG subclass responses, while tolerance induction by unconjugated soluble proteins tends to be targeted on Th1-, but spares Th2-type responses.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuchowski A., McCoy J. R., Palczuk N. C., van Es T., Davis F. F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977 Jun 10;252(11):3582–3586. [PubMed] [Google Scholar]
- Abuchowski A., van Es T., Palczuk N. C., Davis F. F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977 Jun 10;252(11):3578–3581. [PubMed] [Google Scholar]
- Adorini L., Appella E., Doria G., Nagy Z. A. Mechanisms influencing the immunodominance of T cell determinants. J Exp Med. 1988 Dec 1;168(6):2091–2104. doi: 10.1084/jem.168.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech J. T., Bainbridge T., Thompson S. J. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J Immunol Methods. 1997 Jul 14;205(2):163–168. doi: 10.1016/s0022-1759(97)00072-0. [DOI] [PubMed] [Google Scholar]
- Burstein H. J., Abbas A. K. In vivo role of interleukin 4 in T cell tolerance induced by aqueous protein antigen. J Exp Med. 1993 Feb 1;177(2):457–463. doi: 10.1084/jem.177.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein H. J., Shea C. M., Abbas A. K. Aqueous antigens induce in vivo tolerance selectively in IL-2- and IFN-gamma-producing (Th1) cells. J Immunol. 1992 Jun 15;148(12):3687–3691. [PubMed] [Google Scholar]
- Chen R. H., Abuchowski A., Van Es T., Palczuk N. C., Davis F. F. Properties of two urate oxidases modified by the covalent attachment of poly(ethylene glycol). Biochim Biophys Acta. 1981 Aug 13;660(2):293–298. doi: 10.1016/0005-2744(81)90173-x. [DOI] [PubMed] [Google Scholar]
- Chen Y., Inobe J., Weiner H. L. Induction of oral tolerance to myelin basic protein in CD8-depleted mice: both CD4+ and CD8+ cells mediate active suppression. J Immunol. 1995 Jul 15;155(2):910–916. [PubMed] [Google Scholar]
- Durie F. H., Fava R. A., Foy T. M., Aruffo A., Ledbetter J. A., Noelle R. J. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science. 1993 Sep 3;261(5126):1328–1330. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Garside P., Steel M., Worthey E. A., Satoskar A., Alexander J., Bluethmann H., Liew F. Y., Mowat A. M. T helper 2 cells are subject to high dose oral tolerance and are not essential for its induction. J Immunol. 1995 Jun 1;154(11):5649–5655. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Griggs N. D., Agersborg S. S., Noelle R. J., Ledbetter J. A., Linsley P. S., Tung K. S. The relative contribution of the CD28 and gp39 costimulatory pathways in the clonal expansion and pathogenic acquisition of self-reactive T cells. J Exp Med. 1996 Mar 1;183(3):801–810. doi: 10.1084/jem.183.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Ito H. O., So T., Ueda T., Imoto T., Koga T. Prevention of collagen-induced arthritis (CIA) by treatment with polyethylene glycol-conjugated type II collagen; distinct tolerogenic property of the conjugated collagen from the native one. Clin Exp Immunol. 1997 May;108(2):213–219. doi: 10.1046/j.1365-2249.1997.3721269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. J., van den Hoek A. E., van de Putte L. B., van den Berg W. B. Anergy of antigen-specific T lymphocytes is a potent mechanism of intravenously induced tolerance. Immunology. 1994 Jun;82(2):294–300. [PMC free article] [PubMed] [Google Scholar]
- Khoury S. J., Akalin E., Chandraker A., Turka L. A., Linsley P. S., Sayegh M. H., Hancock W. W. CD28-B7 costimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol. 1995 Nov 15;155(10):4521–4524. [PubMed] [Google Scholar]
- Knoerzer D. B., Karr R. W., Schwartz B. D., Mengle-Gaw L. J. Collagen-induced arthritis in the BB rat. Prevention of disease by treatment with CTLA-4-Ig. J Clin Invest. 1995 Aug;96(2):987–993. doi: 10.1172/JCI118146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodera Y., Tanaka H., Matsushima A., Inada Y. Chemical modification of L-asparaginase with a comb-shaped copolymer of polyethylene glycol derivative and maleic anhydride. Biochem Biophys Res Commun. 1992 Apr 15;184(1):144–148. doi: 10.1016/0006-291x(92)91170-u. [DOI] [PubMed] [Google Scholar]
- Kresina T. F., Finegan C. K. Restricted expression of anti-type II collagen antibody isotypes in mice suppressed for collagen-induced arthritis. Ann Rheum Dis. 1986 Jan;45(1):60–66. doi: 10.1136/ard.45.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. Y., Sehon A. H. Abrogation of reaginic antibodies with modified allergens. Nature. 1977 Jun 16;267(5612):618–619. doi: 10.1038/267618a0. [DOI] [PubMed] [Google Scholar]
- Lee W. Y., Sehon A. H. Suppression of reaginic antibodies with modified allergens. I. Reduction in allergenicity of protein allergens by conjugation to polyethylene glycol. Int Arch Allergy Appl Immunol. 1978;56(2):159–170. doi: 10.1159/000232019. [DOI] [PubMed] [Google Scholar]
- Nakata Y., Matsuda K., Uzawa A., Nomura M., Akashi M., Suzuki G. Administration of recombinant human IL-1 by Staphylococcus enterotoxin B prevents tolerance induction in vivo. J Immunol. 1995 Nov 1;155(9):4231–4235. [PubMed] [Google Scholar]
- Romball C. G., Weigle W. O. In vivo induction of tolerance in murine CD4+ cell subsets. J Exp Med. 1993 Nov 1;178(5):1637–1644. doi: 10.1084/jem.178.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayegh M. H., Akalin E., Hancock W. W., Russell M. E., Carpenter C. B., Linsley P. S., Turka L. A. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med. 1995 May 1;181(5):1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Tang A., Judge T. A., Nickoloff B. J., Turka L. A. Suppression of murine allergic contact dermatitis by CTLA4Ig. Tolerance induction of Th2 responses requires additional blockade of CD40-ligand. J Immunol. 1996 Jul 1;157(1):117–125. [PubMed] [Google Scholar]
- Teppler H., Kaplan G., Smith K. A., Montana A. L., Meyn P., Cohn Z. A. Prolonged immunostimulatory effect of low-dose polyethylene glycol interleukin 2 in patients with human immunodeficiency virus type 1 infection. J Exp Med. 1993 Feb 1;177(2):483–492. doi: 10.1084/jem.177.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. E., Dynesius-Trentham R. A., Orav E. J., Combitchi D., Lorenzo C., Sewell K. L., Hafler D. A., Weiner H. L. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993 Sep 24;261(5129):1727–1730. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- Van den Eertwegh A. J., Noelle R. J., Roy M., Shepherd D. M., Aruffo A., Ledbetter J. A., Boersma W. J., Claassen E. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T-B cell interactions. J Exp Med. 1993 Nov 1;178(5):1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Friedman A., Miller A., Khoury S. J., al-Sabbagh A., Santos L., Sayegh M., Nussenblatt R. B., Trentham D. E., Hafler D. A. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- Wilkinson I., Jackson C. J., Lang G. M., Holford-Strevens V., Sehon A. H. Tolerance induction in mice by conjugates of monoclonal immunoglobulins and monomethoxypolyethylene glycol. Transfer of tolerance by T cells and by T cell extracts. J Immunol. 1987 Jul 15;139(2):326–331. [PubMed] [Google Scholar]


