Abstract
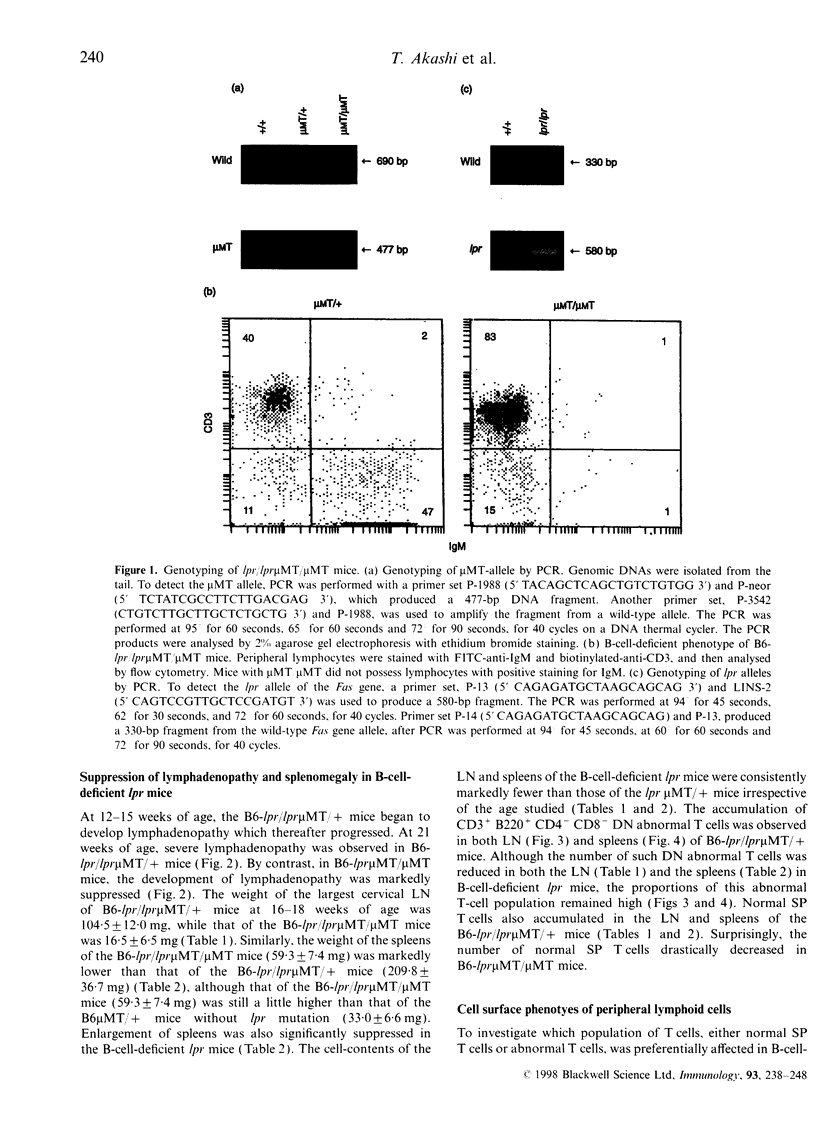
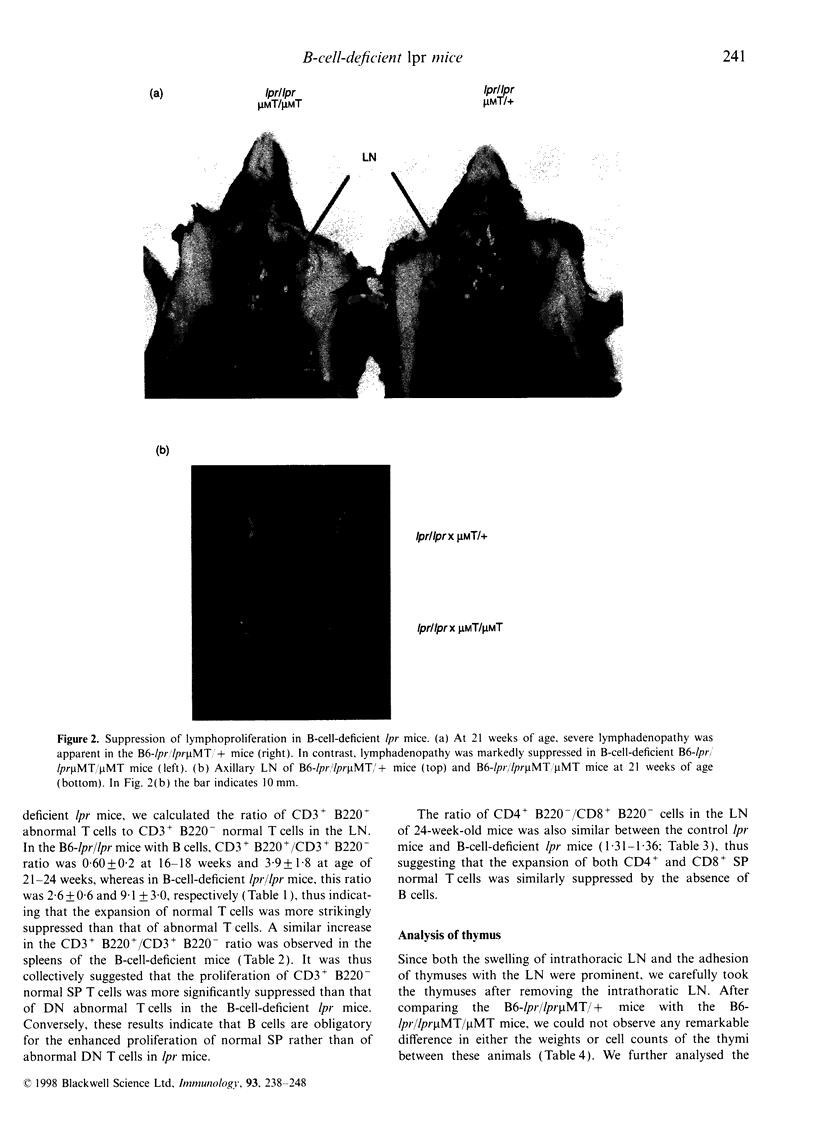
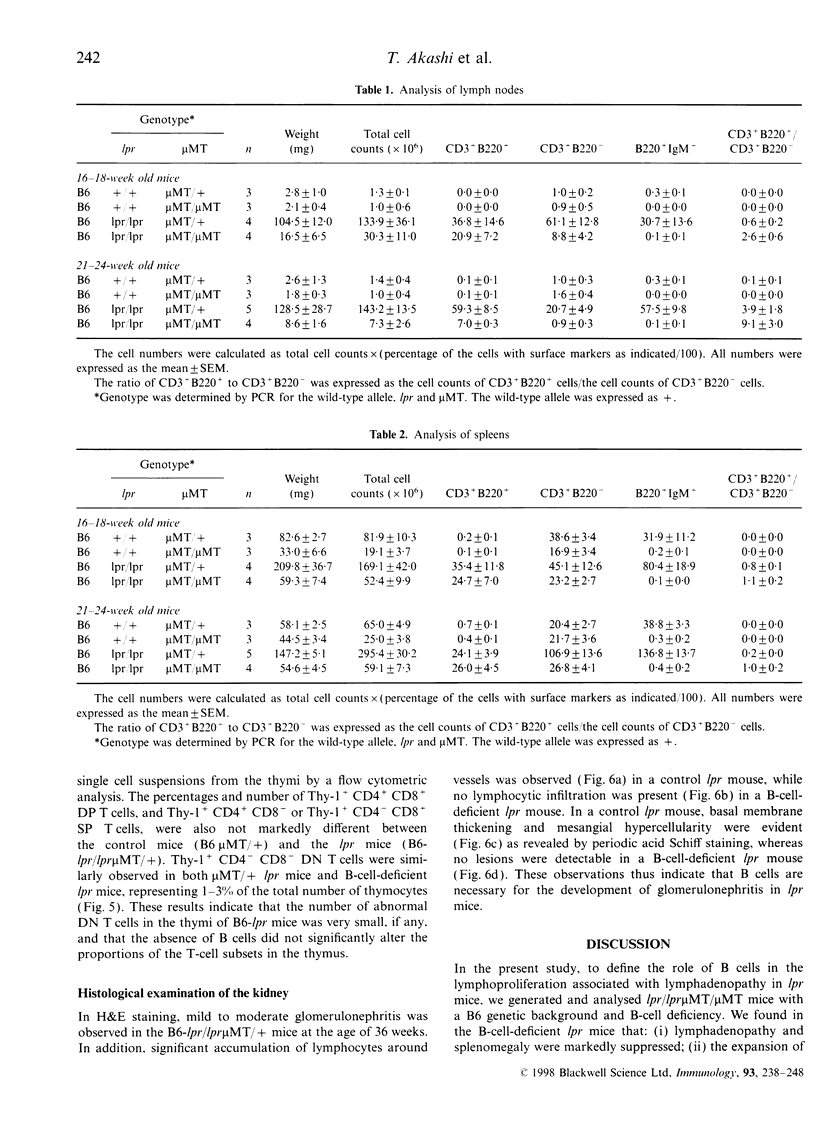
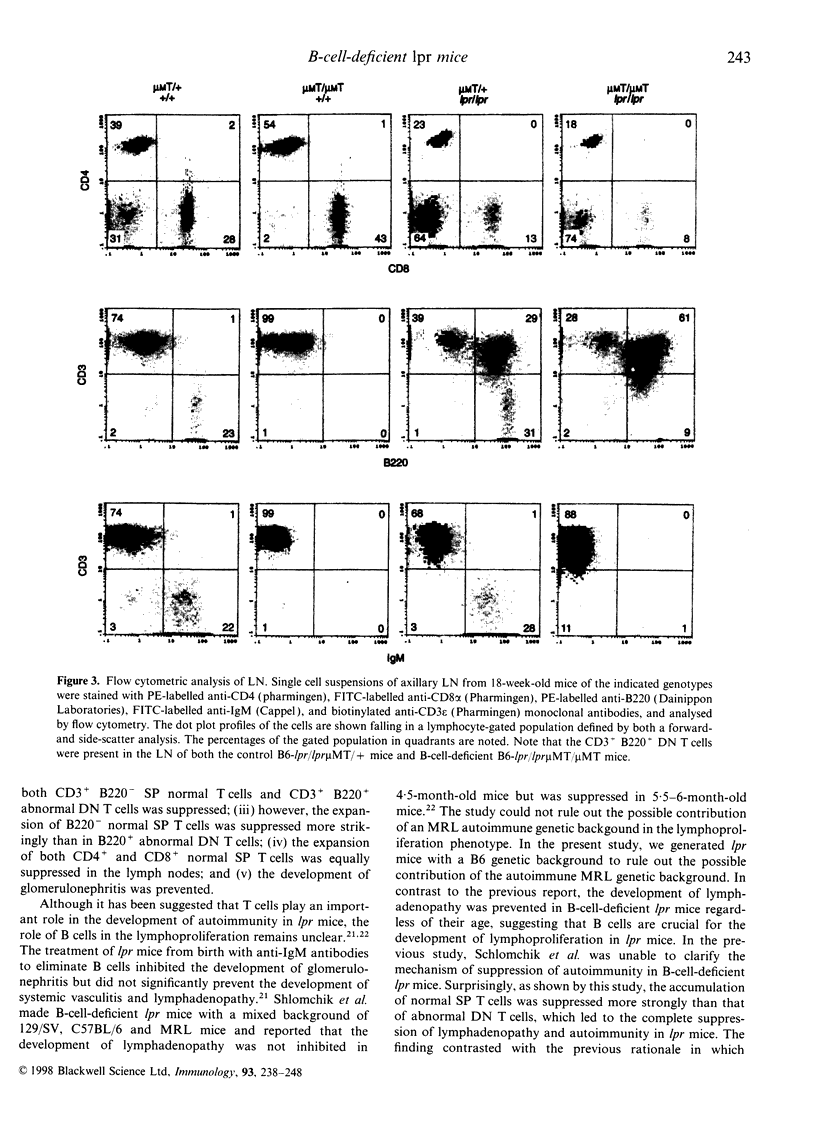
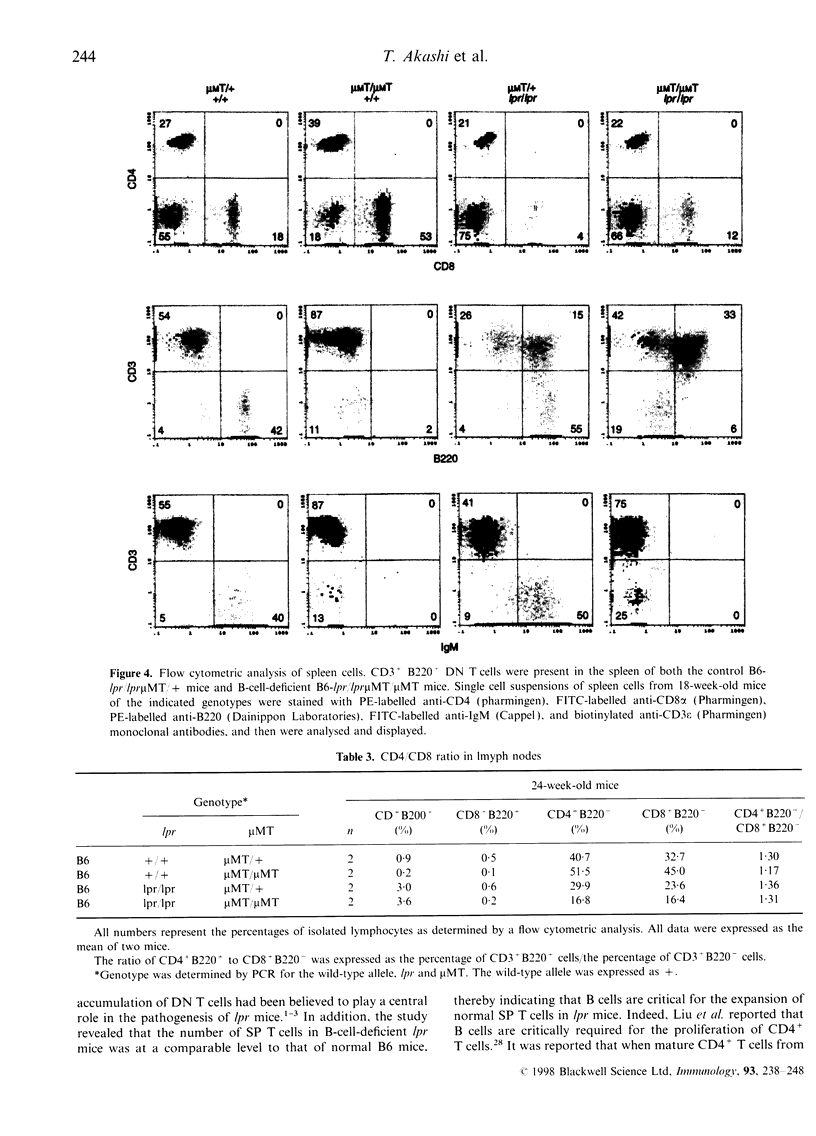
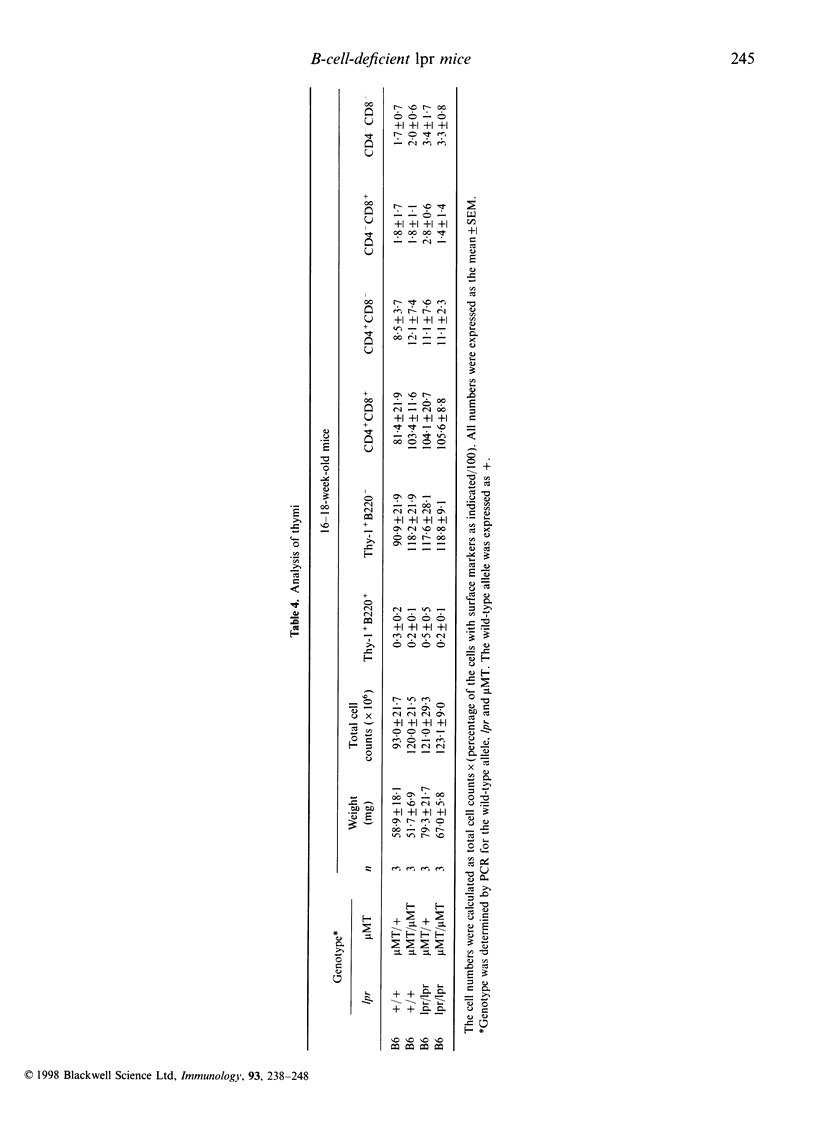
It is known that lpr mice develop systemic lymphadenopathy and lupus erythematosus-like autoimmune disease that are associated with the accumulation of CD4- CD8- (double-negative; DN) CD3+ B220+ abnormal T cells as well as normal mature CD4+ or CD8+ single-positive (SP) CD3+ T cells. In order to clarify the role of B cells in the lymphoproliferation and autoimmunity of lpr mice, we created B-cell-deficient C57BL/6 (B6) lpr mice (B6lpr/lpr microMT/microMT) by crossing B6lpr/lpr mice with B6 microMT/microMT mice in which the B-cell development was arrested at pre-B stage owing to a targeted disruption of the immunoglobulin mu heavy-chain gene locus. In the B-cell-deficient B6-lpr mice, both lymphadenopathy and splenomegaly were markedly suppressed. Although the accumulation of both CD3+ B220- SP normal T cells and CD3+ B220+ DN abnormal T cells was inhibited in the B-cell-deficient lpr mice, the decrease in numbers of CD3+ B220- SP normal T cells occurred more strikingly than that of the CD3+ B220+ DN abnormal T cells. Glomerulonephritis did not develop in the B-cell-deficient lpr mice over 40 weeks. The present results indicate that the B cells thus play a crucial role in the extensive proliferation of normal CD3+ B220- mature SP T cells rather than the accumulation of abnormal DN T cells.
Full text
PDF

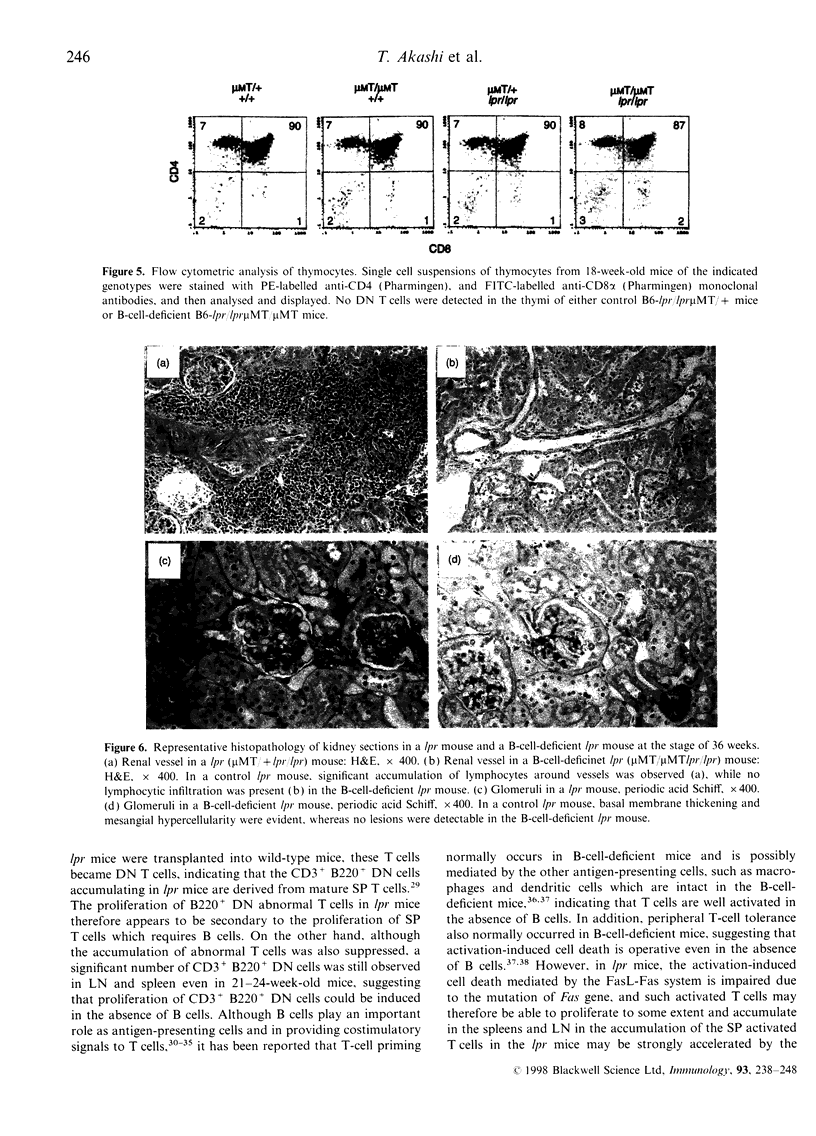


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Watanabe-Fukunaga R., Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi T., Nagafuchi S., Anzai K., Kondo S., Kitamura D., Wakana S., Ono J., Kikuchi M., Niho Y., Watanabe T. Direct evidence for the contribution of B cells to the progression of insulitis and the development of diabetes in non-obese diabetic mice. Int Immunol. 1997 Aug;9(8):1159–1164. doi: 10.1093/intimm/9.8.1159. [DOI] [PubMed] [Google Scholar]
- Cerny A., Kimoto M., Hügin A. W., Merino R., Izui S. Anti-IgM treatment of C57BL/6-1pr/1pr mice: depletion of B cells reduces 1pr gene-induced lymphoproliferation and mononuclear cell vasculitis. Clin Exp Immunol. 1989 Jul;77(1):124–129. [PMC free article] [PubMed] [Google Scholar]
- Chu J. L., Drappa J., Parnassa A., Elkon K. B. The defect in Fas mRNA expression in MRL/lpr mice is associated with insertion of the retrotransposon, ETn. J Exp Med. 1993 Aug 1;178(2):723–730. doi: 10.1084/jem.178.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. L., Eisenberg R. A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Eastcott J. W., Schwartz R. S., Datta S. K. Genetic analysis of the inheritance of B cell hyperactivity in relation to the development of autoantibodies and glomerulonephritis in NZB x SWR crosses. J Immunol. 1983 Nov;131(5):2232–2239. [PubMed] [Google Scholar]
- Epstein M. M., Di Rosa F., Jankovic D., Sher A., Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995 Oct 1;182(4):915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese T., Davidson W. F. In CD8+ T cell-deficient lpr/lpr mice, CD4+B220+ and CD4+B220- T cells replace B220+ double-negative T cells as the predominant populations in enlarged lymph nodes. J Immunol. 1995 May 15;154(10):4986–4995. [PubMed] [Google Scholar]
- Hang L., Theofilopoulos A. N., Balderas R. S., Francis S. J., Dixon F. J. The effect of thymectomy on lupus-prone mice. J Immunol. 1984 Apr;132(4):1809–1813. [PubMed] [Google Scholar]
- Herron L. R., Eisenberg R. A., Roper E., Kakkanaiah V. N., Cohen P. L., Kotzin B. L. Selection of the T cell receptor repertoire in Lpr mice. J Immunol. 1993 Oct 1;151(7):3450–3459. [PubMed] [Google Scholar]
- Izui S., Kelley V. E., Masuda K., Yoshida H., Roths J. B., Murphy E. D. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J Immunol. 1984 Jul;133(1):227–233. [PubMed] [Google Scholar]
- Jevnikar A. M., Grusby M. J., Glimcher L. H. Prevention of nephritis in major histocompatibility complex class II-deficient MRL-lpr mice. J Exp Med. 1994 Apr 1;179(4):1137–1143. doi: 10.1084/jem.179.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Hirano T., Kakinuma M., Uede T. Transcriptional repression and differential splicing of Fas mRNA by early transposon (ETn) insertion in autoimmune lpr mice. Biochem Biophys Res Commun. 1993 Mar 15;191(2):617–624. doi: 10.1006/bbrc.1993.1262. [DOI] [PubMed] [Google Scholar]
- Koh D. R., Ho A., Rahemtulla A., Fung-Leung W. P., Griesser H., Mak T. W. Murine lupus in MRL/lpr mice lacking CD4 or CD8 T cells. Eur J Immunol. 1995 Sep;25(9):2558–2562. doi: 10.1002/eji.1830250923. [DOI] [PubMed] [Google Scholar]
- Kuchroo V. K., Das M. P., Brown J. A., Ranger A. M., Zamvil S. S., Sobel R. A., Weiner H. L., Nabavi N., Glimcher L. H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995 Mar 10;80(5):707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Liano D., HayGlass K. A., Benacerraf B., Sy M. S., Abbas A. K. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J Immunol. 1988 Jun 1;140(11):3773–3778. [PubMed] [Google Scholar]
- Laouar Y., Ezine S. In vivo CD4+ lymph node T cells from lpr mice generate CD4-CD8-B220+TCR-beta low cells. J Immunol. 1994 Nov 1;153(9):3948–3955. [PubMed] [Google Scholar]
- Lin R. H., Mamula M. J., Hardin J. A., Janeway C. A., Jr Induction of autoreactive B cells allows priming of autoreactive T cells. J Exp Med. 1991 Jun 1;173(6):1433–1439. doi: 10.1084/jem.173.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wu Y., Ramarathinam L., Guo Y., Huszar D., Trounstine M., Zhao M. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int Immunol. 1995 Aug;7(8):1353–1362. doi: 10.1093/intimm/7.8.1353. [DOI] [PubMed] [Google Scholar]
- Mamula M. J., Lin R. H., Janeway C. A., Jr, Hardin J. A. Breaking T cell tolerance with foreign and self co-immunogens. A study of autoimmune B and T cell epitopes of cytochrome c. J Immunol. 1992 Aug 1;149(3):789–795. [PubMed] [Google Scholar]
- Mixter P. F., Russell J. Q., Durie F. H., Budd R. C. Decreased CD4-CD8- TCR-alpha beta + cells in lpr/lpr mice lacking beta 2-microglobulin. J Immunol. 1995 Mar 1;154(5):2063–2074. [PubMed] [Google Scholar]
- Nagata S., Golstein P. The Fas death factor. Science. 1995 Mar 10;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Phillips J. A., Romball C. G., Hobbs M. V., Ernst D. N., Shultz L., Weigle W. O. CD4+ T cell activation and tolerance induction in B cell knockout mice. J Exp Med. 1996 Apr 1;183(4):1339–1344. doi: 10.1084/jem.183.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveche E. S., Novotny E. A., Hansen C. T., Tjio J. H., Steinberg A. D. Genetic studies in NZB mice. V. Recombinant inbred lines demonstrate that separate genes control autoimmune phenotype. J Exp Med. 1981 May 1;153(5):1187–1197. doi: 10.1084/jem.153.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron Y., Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987 May 1;138(9):2848–2856. [PubMed] [Google Scholar]
- Russell J. H., Rush B., Weaver C., Wang R. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4409–4413. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M. J., Madaio M. P., Ni D., Trounstein M., Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994 Oct 1;180(4):1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman C. L., Marshall J. D., Von Boehmer H. Transgenic T cell receptor interactions in the lymphoproliferative and autoimmune syndromes of lpr and gld mutant mice. Eur J Immunol. 1992 Feb;22(2):499–504. doi: 10.1002/eji.1830220231. [DOI] [PubMed] [Google Scholar]
- Smith B. R., Hall R. Thyroid-stimulating immunoglobulins in Graves' disease. Lancet. 1974 Aug 24;2(7878):427–431. doi: 10.1016/s0140-6736(74)91815-7. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Roths J. B., Murphy E. D., Steinberg R. T., Raveche E. S. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J Immunol. 1980 Aug;125(2):871–873. [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Etiopathogenesis of murine SLE. Immunol Rev. 1981;55:179–216. doi: 10.1111/j.1600-065x.1981.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Thompson C. B. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell. 1995 Jun 30;81(7):979–982. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- Vella A. T., Scherer M. T., Schultz L., Kappler J. W., Marrack P. B cells are not essential for peripheral T-cell tolerance. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):951–955. doi: 10.1073/pnas.93.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Watson M. L., Rao J. K., Gilkeson G. S., Ruiz P., Eicher E. M., Pisetsky D. S., Matsuzawa A., Rochelle J. M., Seldin M. F. Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J Exp Med. 1992 Dec 1;176(6):1645–1656. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofsy D., Ledbetter J. A., Hendler P. L., Seaman W. E. Treatment of murine lupus with monoclonal anti-T cell antibody. J Immunol. 1985 Feb;134(2):852–857. [PubMed] [Google Scholar]
- Wu J., Zhou T., He J., Mountz J. D. Autoimmune disease in mice due to integration of an endogenous retrovirus in an apoptosis gene. J Exp Med. 1993 Aug 1;178(2):461–468. doi: 10.1084/jem.178.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]