Abstract
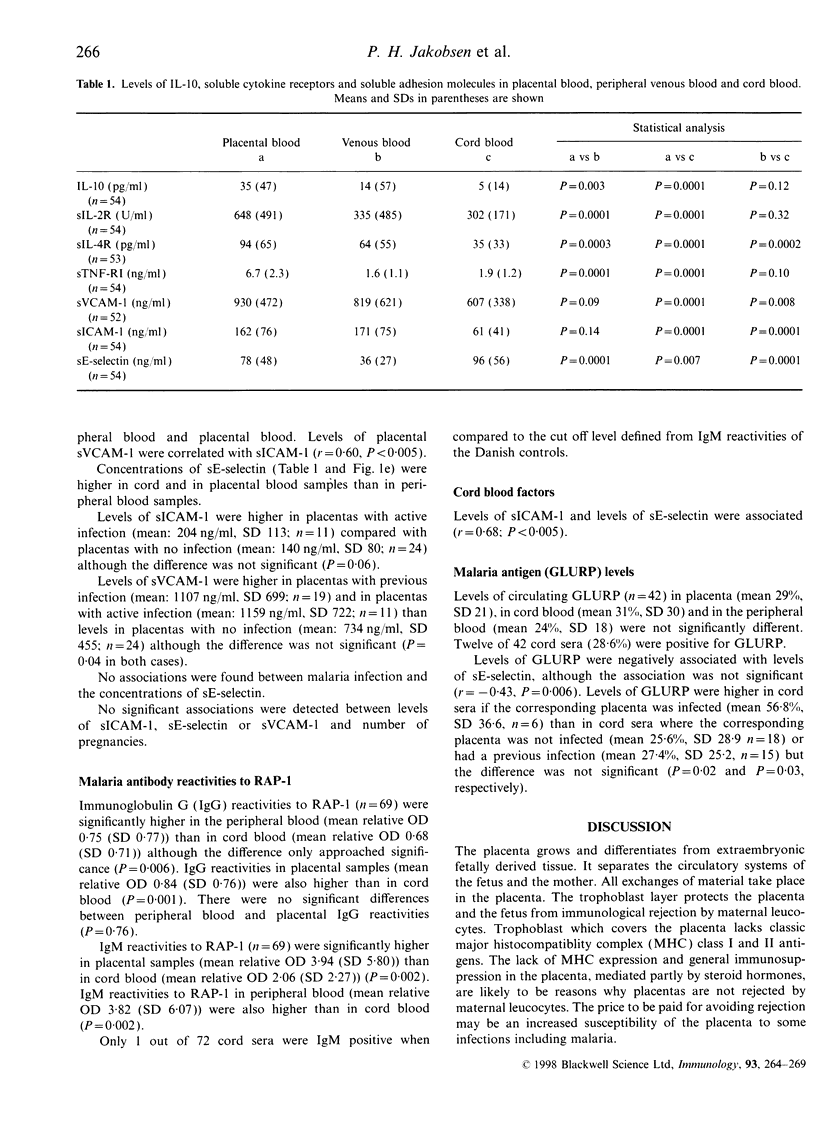
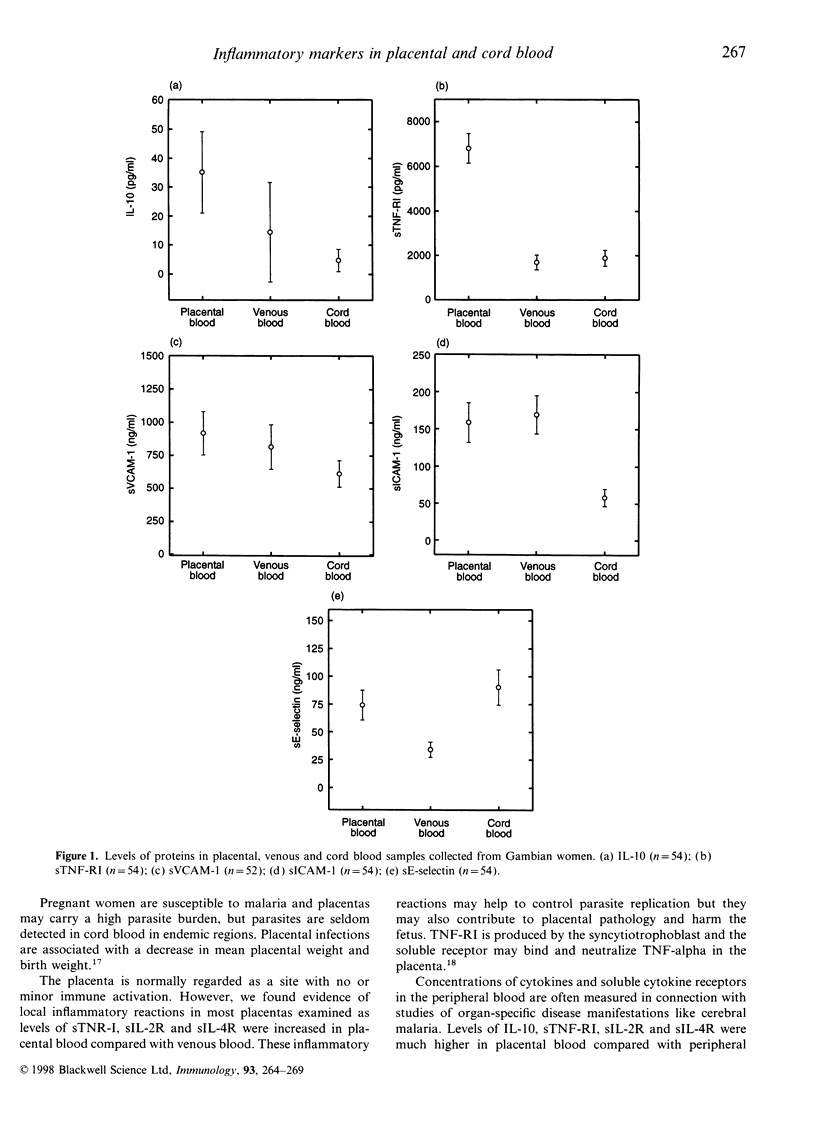
To better understand reasons for increased susceptibility to malaria in pregnancy; and the interrelationships between maternal malaria, local immune reactions and the development of the fetus, concentrations of soluble interleukin-10 (IL-10), cytokine receptors, adhesion molecules, a Plasmodium falciparum protein, glutamate-rich protein (GLURP) and antibodies to P. falciparum rhoptry-associated protein-1 were measured among 105 Gambian women and their neonates. Peripheral blood concentrations of IL-10, soluble cytokine receptors and soluble adhesion molecules were found to be different from those concentrations measured in the placenta. Markers of inflammatory reactions: IL-10, sIL-2R, sIL-4R, and soluble tumour necrosis factor receptor I (sTNF-RI) were found in high concentrations in the placenta, indicating that inflammatory reactions take place in the placenta which has been regarded as an immunoprivileged site. Concentrations of soluble vascular cell adhesion molecule-1 (sVCAM-1) and soluble intracellular adhesion molecule-1 (sICAM-1), potential adhesion receptors for malaria parasites, were associated with an active P. falciparum infection in the placenta although the associations did not reach significance. P. falciparum exoantigen, GLURP, was detected in cord blood indicating transplacental passage of malarial antigens. Concentrations of E-selectin were higher in cord blood samples compared with peripheral blood samples. This appeared to be associated with development of cord endothelial cells and not with P. falciparum infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berendt A. R., Simmons D. L., Tansey J., Newbold C. I., Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989 Sep 7;341(6237):57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- Borre M. B., Dziegiel M., Høgh B., Petersen E., Rieneck K., Riley E., Meis J. F., Aikawa M., Nakamura K., Harada M. Primary structure and localization of a conserved immunogenic Plasmodium falciparum glutamate rich protein (GLURP) expressed in both the preerythrocytic and erythrocytic stages of the vertebrate life cycle. Mol Biochem Parasitol. 1991 Nov;49(1):119–131. doi: 10.1016/0166-6851(91)90135-s. [DOI] [PubMed] [Google Scholar]
- Brabin B. J. An analysis of malaria in pregnancy in Africa. Bull World Health Organ. 1983;61(6):1005–1016. [PMC free article] [PubMed] [Google Scholar]
- Bulmer J. N., Rasheed F. N., Morrison L., Francis N., Greenwood B. M. Placental malaria. II. A semi-quantitative investigation of the pathological features. Histopathology. 1993 Mar;22(3):219–225. doi: 10.1111/j.1365-2559.1993.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Druilhe P., Monjour L., Gentilini M. Passage transplacentaire des antigènes solubles plasmodiaux. Induction d'une tolérance immunitaire spécifique. Nouv Presse Med. 1976 May 29;5(22):1430–1431. [PubMed] [Google Scholar]
- Forsthuber T., Yip H. C., Lehmann P. V. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996 Mar 22;271(5256):1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- Fried M., Duffy P. E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996 Jun 7;272(5267):1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- Garin Y. J., Blot P., Walter P., Pinon J. M., Vernes A. Placentopathies palustres. Aspects parasitologiques, cliniques et immunologiques. Arch Fr Pediatr. 1985 Dec;42 (Suppl 2):917–920. [PubMed] [Google Scholar]
- Jakobsen P. H., Lemnge M. M., Abu-Zeid Y. A., Msangeni H. A., Salum F. M., Mhina J. I., Akida J. A., Ruta A. S., Ronn A. M., Heegaard P. M. Immunoglobulin G reactivities to rhoptry-associated protein-1 associated with decreased levels of Plasmodium falciparum parasitemia in Tanzanian children. Am J Trop Med Hyg. 1996 Dec;55(6):642–646. doi: 10.4269/ajtmh.1996.55.642. [DOI] [PubMed] [Google Scholar]
- Jakobsen P. H., Morris-Jones S., Rønn A., Hviid L., Theander T. G., Elhassan I. M., Bygbjerg I. C., Greenwood B. M. Increased plasma concentrations of sICAM-1, sVCAM-1 and sELAM-1 in patients with Plasmodium falciparum or P. vivax malaria and association with disease severity. Immunology. 1994 Dec;83(4):665–669. [PMC free article] [PubMed] [Google Scholar]
- Kräling B. M., Razon M. J., Boon L. M., Zurakowski D., Seachord C., Darveau R. P., Mulliken J. B., Corless C. L., Bischoff J. E-selectin is present in proliferating endothelial cells in human hemangiomas. Am J Pathol. 1996 Apr;148(4):1181–1191. [PMC free article] [PubMed] [Google Scholar]
- Kwee L., Baldwin H. S., Shen H. M., Stewart C. L., Buck C., Buck C. A., Labow M. A. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995 Feb;121(2):489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- Menendez C. Malaria during pregnancy: a priority area of malaria research and control. Parasitol Today. 1995 May;11(5):178–183. doi: 10.1016/0169-4758(95)80151-0. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Tegoshi T., Maeno Y., Benjamin C., Ho M., Kan K. E., Thway Y., Win K., Aikawa M., Lobb R. R. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992 Oct 1;176(4):1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed F. N., Bulmer J. N., De Francisco A., Jawla M. F., Jakobsen P. H., Jepson A., Greenwood B. M. Relationships between maternal malaria and malarial immune responses in mothers and neonates. Parasite Immunol. 1995 Jan;17(1):1–10. doi: 10.1111/j.1365-3024.1995.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Rasheed F. N. Maternal infections influence infection susceptibility in childhood. Med Hypotheses. 1994 Feb;42(2):76–80. doi: 10.1016/0306-9877(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Ridley R. G., Takacs B., Lahm H. W., Delves C. J., Goman M., Certa U., Matile H., Woollett G. R., Scaife J. G. Characterisation and sequence of a protective rhoptry antigen from Plasmodium falciparum. Mol Biochem Parasitol. 1990 Jun;41(1):125–134. doi: 10.1016/0166-6851(90)90103-s. [DOI] [PubMed] [Google Scholar]
- Wegmann T. G., Lin H., Guilbert L., Mosmann T. R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993 Jul;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]


