Abstract
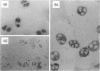
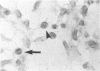
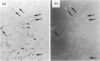
To determine if nitric oxide (NO) is produced by chronically infected human polymorphonuclear leucocytes (PMNs) in vivo, inflamed exudates (periapical exudates: PE) collected from periapical periodontitis patients were examined. Cell-free supernatants and cells were separated by centrifugation. Significant levels of nitrite concentrations were observed in the supernatants. The production of inducible NO synthase (iNOS) in highly purified PMNs derived from PEs was then immunocytochemically determined using rabbit anti-human iNOS antiserum. In vitro, human peripheral blood PMNs (PB-PMNs) isolated from patients were cultured with a combination of Esherichia coli-lipopolysaccharide (LPS), recombinant human interferon-gamma (rhIFN-gamma) and/or interleukin-1 beta (rhIL-1 beta). The stimulated PB-PMNs showed steady-state levels of nitrite. The stimulation of LPS, rhIFN-gamma and rhIL-1 beta showed more NO induction than that of LPS with either IFN-gamma or IL-1 beta, suggesting the synergistic effects of cytokines. Cryostat sections of surgically removed periapical tissues were also immunohistochemically examined for iNOS, IFN-gamma and IL-1 beta. Two-colour immunohistochemistry revealed the interaction of iNOS-producing PMNs and IFN-gamma- or IL-1 beta-producing mononuclear cells. On the basis of these data, we concluded that with the stimulation of inflammatory cytokines derived from mononuclear cells, PMNs can spontaneously produce NO at the site of chronic infection. The present studies are consistent with a hypothesis suggesting that PMNs could be regulated and delicately balanced to produce NO by mononuclear cell-derived cytokines in vivo. NO-producing cells may play a pivotal role in chronic inflammation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina J. E., Abate J. A., Henry W. L., Jr Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J Immunol. 1991 Jul 1;147(1):144–148. [PubMed] [Google Scholar]
- Albina J. E., Cui S., Mateo R. B., Reichner J. S. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993 Jun 1;150(11):5080–5085. [PubMed] [Google Scholar]
- Bauer H., Jung T., Tsikas D., Stichtenoth D. O., Frölich J. C., Neumann C. Nitric oxide inhibits the secretion of T-helper 1- and T-helper 2-associated cytokines in activated human T cells. Immunology. 1997 Feb;90(2):205–211. doi: 10.1046/j.1365-2567.1997.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Farrell A. J., Blake D. R., Palmer R. M., Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992 Nov;51(11):1219–1222. doi: 10.1136/ard.51.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro I. M., Barja-Fidalgo C., Cunha F. Q., Ferreira S. H. The involvement of nitric oxide in the anti-Candida albicans activity of rat neutrophils. Immunology. 1996 Oct;89(2):295–300. doi: 10.1046/j.1365-2567.1996.d01-742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S. S., Billiar T., Curran R. D., Zdziarski U. E., Simmons R. L., Basford R. E. Inhibition of chemotaxis Ng-monomethyl-L-arginine: a role for cyclic GMP. Blood. 1989 Nov 1;74(6):1885–1887. [PubMed] [Google Scholar]
- Klebanoff S. J., Nathan C. F. Nitrite production by stimulated human polymorphonuclear leukocytes supplemented with azide and catalase. Biochem Biophys Res Commun. 1993 Nov 30;197(1):192–196. doi: 10.1006/bbrc.1993.2459. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Sakurai H., Kohsaka H., Liu M. F., Higashiyama H., Hirata Y., Kanno K., Saito I., Miyasaka N. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J Clin Invest. 1995 Nov;96(5):2357–2363. doi: 10.1172/JCI118292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder H., Ney P., Woditsch I., Schrör K. Cyclic GMP mediates SIN-1-induced inhibition of human polymorphonuclear leukocytes. Eur J Pharmacol. 1990 Jul 3;182(2):211–218. doi: 10.1016/0014-2999(90)90279-f. [DOI] [PubMed] [Google Scholar]
- Stefanovic-Racic M., Stadler J., Evans C. H. Nitric oxide and arthritis. Arthritis Rheum. 1993 Aug;36(8):1036–1044. doi: 10.1002/art.1780360803. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Pinzon W. L., Strynadka K., Schulz R., Rabinovitch A. Mechanisms of cytokine-induced destruction of rat insulinoma cells: the role of nitric oxide. Endocrinology. 1994 Mar;134(3):1006–1010. doi: 10.1210/endo.134.3.8119136. [DOI] [PubMed] [Google Scholar]
- Takeichi O., Saito I., Tsurumachi T., Moro I., Saito T. Expression of inflammatory cytokine genes in vivo by human alveolar bone-derived polymorphonuclear leukocytes isolated from chronically inflamed sites of bone resorption. Calcif Tissue Int. 1996 Apr;58(4):244–248. doi: 10.1007/BF02508643. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson A. W., Liew F. Y., Severn A., Xu D., McSorley S. J., Garside P., Padron J., Phillips R. S. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur J Immunol. 1994 Apr;24(4):980–984. doi: 10.1002/eji.1830240430. [DOI] [PubMed] [Google Scholar]
- Van Dervort A. L., Yan L., Madara P. J., Cobb J. P., Wesley R. A., Corriveau C. C., Tropea M. M., Danner R. L. Nitric oxide regulates endotoxin-induced TNF-alpha production by human neutrophils. J Immunol. 1994 Apr 15;152(8):4102–4109. [PubMed] [Google Scholar]
- Wei X. Q., Charles I. G., Smith A., Ure J., Feng G. J., Huang F. P., Xu D., Muller W., Moncada S., Liew F. Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995 Jun 1;375(6530):408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Yan L., Vandivier R. W., Suffredini A. F., Danner R. L. Human polymorphonuclear leukocytes lack detectable nitric oxide synthase activity. J Immunol. 1994 Aug 15;153(4):1825–1834. [PubMed] [Google Scholar]