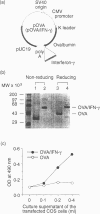
Abstract
The preferential differentiation of T helper (Th) cells to Th1 or Th2 subsets is important with respect to susceptibility or resistance to particular infections, or to autoimmune diseases and allergic diseases. To more effectively drive immune responses toward antigen-specific Th1 responses, we constructed a mammalian expression vector (pOVA/IFN-gamma) carrying a hybrid gene in which the ovalbumin (OVA) (a model antigen) cDNA was covalently linked to murine interferon-gamma (IFN-gamma) cDNA. Intramuscular injection of BALB/c mice with the pOVA/IFN-gamma DNA increased both the production of OVA-specific IFN-gamma by CD4+ T cells and the ratio of anti-OVA immunoglobulin G (IgG) 2a to IgG1 isotypes, while the injection with the pOVA alone, or with the mixture of the pOVA and pIFN-gamma, caused no or little increase. Furthermore, the OVA-specific, Th1 immune responses were dramatically augmented by multiple injections with the pOVA/IFN-gamma DNA. These studies indicate that the direct linkage of an OVA gene to an IFN-gamma gene in the expression plasmid is required for efficiently confining the Th1 effects of IFN-gamma to the OVA-specific cells, and the linkage effect of the OVA/IFN-gamma DNA can be potentiated by multiple vaccination.
Full text
PDF
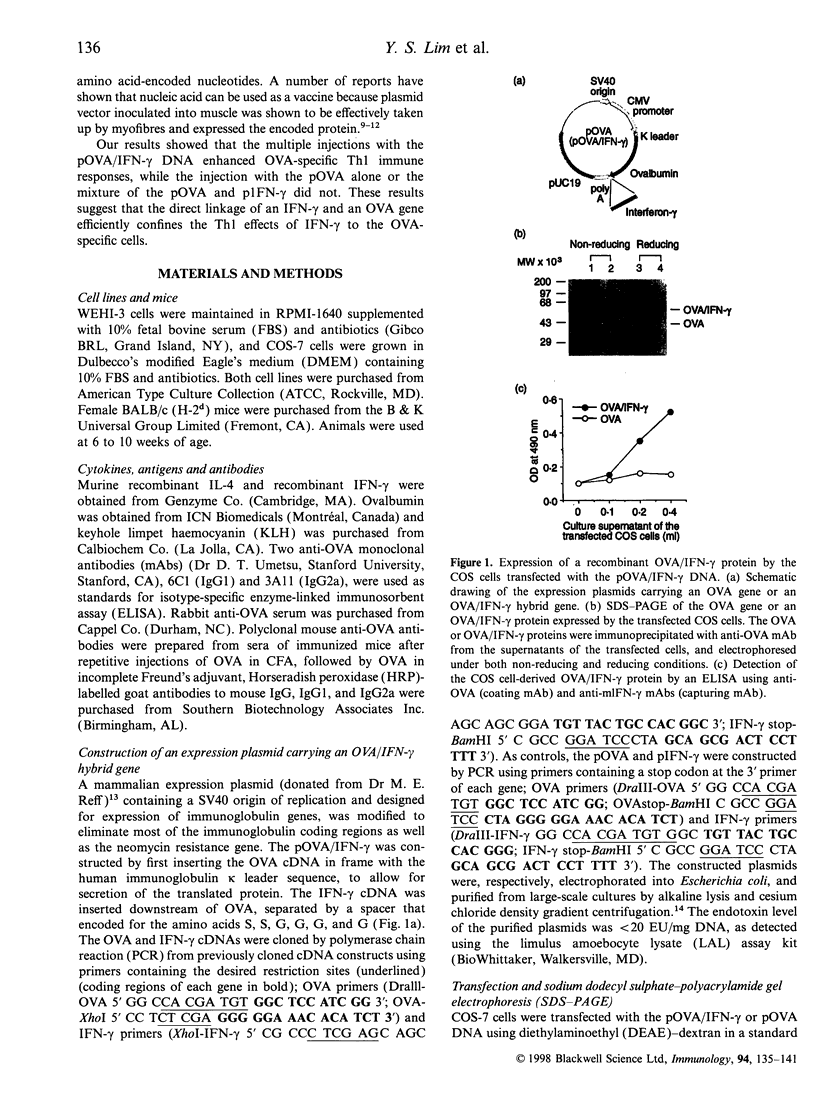

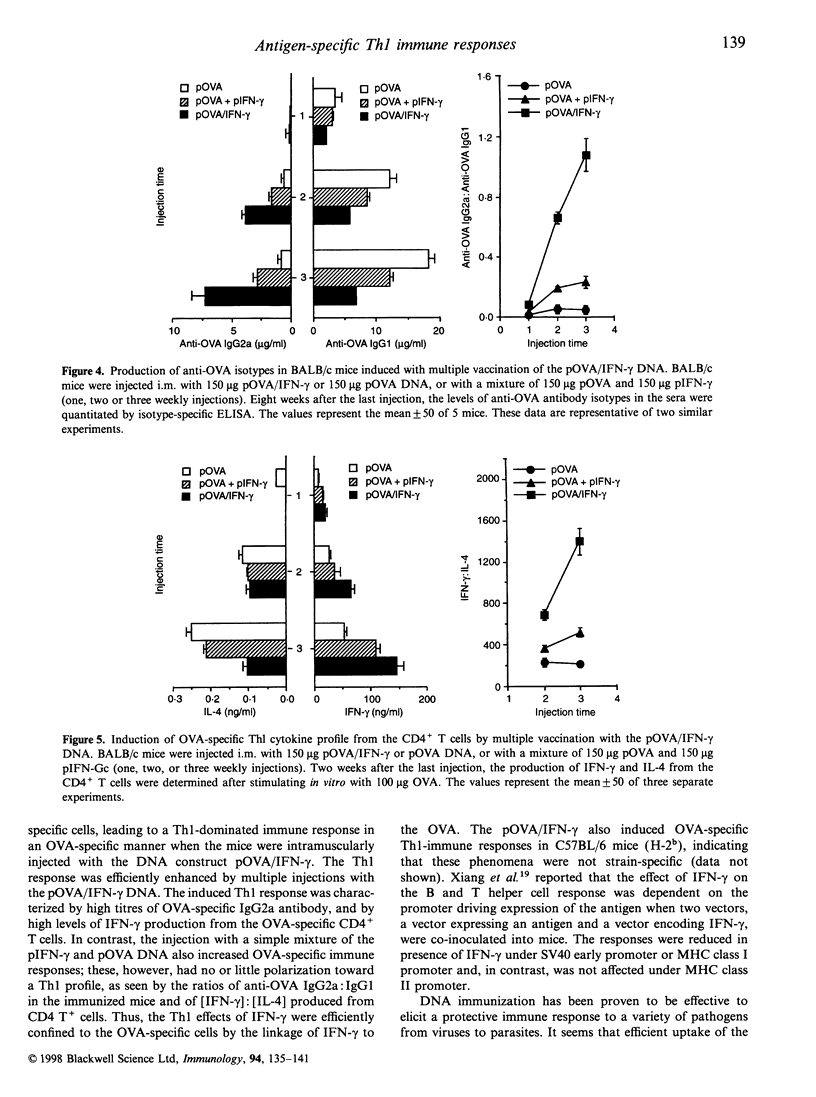


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chow Y. H., Huang W. L., Chi W. K., Chu Y. D., Tao M. H. Improvement of hepatitis B virus DNA vaccines by plasmids coexpressing hepatitis B surface antigen and interleukin-2. J Virol. 1997 Jan;71(1):169–178. doi: 10.1128/jvi.71.1.169-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy I. M., DeKruyff R. H., Tate K. M., Cao Z. A., Moore T. A., Umetsu D. T., Jones P. P. Novel genetic regulation of T helper 1 (Th1)/Th2 cytokine production and encephalitogenicity in inbred mouse strains. J Exp Med. 1997 Feb 3;185(3):439–451. doi: 10.1084/jem.185.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant S. L., Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- Danko I., Wolff J. A. Direct gene transfer into muscle. Vaccine. 1994 Dec;12(16):1499–1502. doi: 10.1016/0264-410x(94)90072-8. [DOI] [PubMed] [Google Scholar]
- DeKruyff R. H., Fang Y., Wolf S. F., Umetsu D. T. IL-12 inhibits IL-4 synthesis in keyhole limpet hemocyanin-primed CD4+ T cells through an effect on antigen-presenting cells. J Immunol. 1995 Mar 15;154(6):2578–2587. [PubMed] [Google Scholar]
- Gilboa E. Immunotherapy of cancer with genetically modified tumor vaccines. Semin Oncol. 1996 Feb;23(1):101–107. [PubMed] [Google Scholar]
- Gillies S. D., Young D., Lo K. M., Roberts S. Biological activity and in vivo clearance of antitumor antibody/cytokine fusion proteins. Bioconjug Chem. 1993 May-Jun;4(3):230–235. doi: 10.1021/bc00021a008. [DOI] [PubMed] [Google Scholar]
- Gorham J. D., Güler M. L., Steen R. G., Mackey A. J., Daly M. J., Frederick K., Dietrich W. F., Murphy K. M. Genetic mapping of a murine locus controlling development of T helper 1/T helper 2 type responses. Proc Natl Acad Sci U S A. 1996 Oct 29;93(22):12467–12472. doi: 10.1073/pnas.93.22.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Street N. F., Budd R., O'Garra A., Fong T. A., Bond M. W., Moore K. W., Sher A., Fiorentino D. F. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol Rev. 1991 Oct;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Pertmer T. M., Roberts T. R., Haynes J. R. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996 Sep;70(9):6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reff M. E., Carner K., Chambers K. S., Chinn P. C., Leonard J. E., Raab R., Newman R. A., Hanna N., Anderson D. R. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994 Jan 15;83(2):435–445. [PubMed] [Google Scholar]
- Ricci M., Matucci A., Rossi O. IL-4 as a key factor influencing the development of allergen-specific Th2-like cells in atopic individuals. J Investig Allergol Clin Immunol. 1997 May-Jun;7(3):144–150. [PubMed] [Google Scholar]
- Romagnani S., Parronchi P., D'Elios M. M., Romagnani P., Annunziato F., Piccinni M. P., Manetti R., Sampognaro S., Mavilia C., De Carli M. An update on human Th1 and Th2 cells. Int Arch Allergy Immunol. 1997 May-Jul;113(1-3):153–156. doi: 10.1159/000237532. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996 Sep;80(3 Pt 1):225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- Sher A., Gazzinelli R. T., Oswald I. P., Clerici M., Kullberg M., Pearce E. J., Berzofsky J. A., Mosmann T. R., James S. L., Morse H. C., 3rd Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992 Jun;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Wang B., Ugen K. E., Srikantan V., Agadjanyan M. G., Dang K., Refaeli Y., Sato A. I., Boyer J., Williams W. V., Weiner D. B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. J. Improved gene transfer by direct plasmid injection associated with regeneration in mouse skeletal muscle. FEBS Lett. 1993 Oct 11;332(1-2):179–182. doi: 10.1016/0014-5793(93)80508-r. [DOI] [PubMed] [Google Scholar]
- Whitton J. L., Yokoyama M. Proteins expressed by DNA vaccines induce both local and systemic immune responses. Ann N Y Acad Sci. 1996 Oct 25;797:196–206. doi: 10.1111/j.1749-6632.1996.tb52961.x. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Xiang Z. Q., He Z., Wang Y., Ertl H. C. The effect of interferon-gamma on genetic immunization. Vaccine. 1997 Jun;15(8):896–898. doi: 10.1016/s0264-410x(96)00269-1. [DOI] [PubMed] [Google Scholar]
- Xu D., Liew F. Y. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63, of L. major. Immunology. 1995 Feb;84(2):173–176. [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M., Hassett D. E., Zhang J., Whitton J. L. DNA immunization can stimulate florid local inflammation, and the antiviral immunity induced varies depending on injection site. Vaccine. 1997 Apr;15(5):553–560. doi: 10.1016/s0264-410x(97)00213-2. [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Zhang J., Whitton J. L. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J Virol. 1995 Apr;69(4):2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]