Abstract
The expression of interleukin-2 (IL-2) has been implicated in the modulation of the outcome of ocular infection with herpes simplex virus type 1 (HSV-1); however, its effects remain controversial. To clarify the role of IL-2, we constructed a recombinant HSV-1 (HSV-IL-2) that expresses two copies of the murine IL-2 gene under the control of the latency-associated transcript (LAT) promoter of HSV-1 in a LAT-negative virus. In tissue culture, the replication of the HSV-IL-2 was 100-fold lower than that of the wild-type virus at a low multiplicity of infection (MOI). Addition of recombinant anti-IL-2 polyclonal antibody markedly enhanced HSV-IL-2 replication in tissue culture. In the 7-day period after ocular infection of BALB/c mice, the replication of HSV-IL-2 was significantly lower than that of wild-type virus in tear cultures, whole eyes, and brain, but was equivalent to wild-type replication in the trigeminal ganglia. Ocular challenge of BALB/c mice with HSV-IL-2 alone, at an MOI that resulted in only 13% survival when parental virus was used, was associated with 90% survival. This decrease in virulence was further shown to be attributable to the expression of IL-2 by coinfection of mice with HSV-IL-2 and the parental virus. This resulted in a decrease in virulence of the parental virus (5% survival when administered alone versus 50% survival on coinfection with HSV-IL-2). The survival of HSV-IL-2-infected mice was compromised by depletion of either IL-2, CD4+, or CD8+ T cells (50% survival) and abolished completely by depletion of both T-cell subtypes. Moreover, depletion of CD4+ T cells, CD8+ T cells, or both increased the titers of HSV-IL-2 in the tears, eyes, trigeminal ganglia, and brains of infected mice, so that titers were equivalent to or higher than that of the parental virus. These results suggest that IL-2 expression by recombinant HSV-1 reduces virulence and that depletion of IL-2 or T cells increases virulence in HSV-1-infected mice.
Herpes simplex virus (HSV) infection is one of the most common serious viral eye infections in the United States and is a major cause of virus-induced blindness (7, 39). Subsequent to the primary HSV-1 infection, the virus replicates in the eyes. The damage to the corneal tissue associated with the infection is incurred as a direct consequence of the HSV-1 infection of the cells or secondary to the effects of the cytokines and chemokines that are released from cells infiltrating the cornea in response to the infection (9, 14, 18). Typically, the virus is cleared completely within 7 to 10 days of ocular infection (11-13, 17, 18), with delayed clearance resulting in protracted and more extensive ocular disease. Both a rapid reduction in the initial virus load in the eye and an acceleration of the subsequent clearance of the virus should have a major impact on the prevention of latency and recurrent infections, thereby averting loss of vision.
The potential role of interleukin-2 (IL-2), an inflammatory cytokine involved in the growth and differentiation of lymphocytes (27, 36), in the protective responses of the host to this infection is of great interest but remains controversial. In previous studies, we used immunohistochemical analysis to demonstrate that reduced replication of HSV-1 in the eye is correlated with the presence of IL-2 in the cornea (11, 18). Furthermore, we have shown that IL-2−/− nullizygous mice, which are deficient in IL-2 production, are more sensitive to ocular infection than IL-4−/− nullizygous mice, which are deficient in IL-4 production (13). Other groups have investigated the role of IL-2 in the treatment and control of HSV-1 infection by using IL-2 depletion (20), recombinant IL-2 administration (33, 38), recombinant vaccinia virus vectors expressing IL-2 (2), and immunization with IL-2 DNA (6).
These studies have generated conflicting results as to whether IL-2 protects against HSV-1 infection. Some suggest that IL-2 may play a pathogenic role (3, 20), another suggests that it may play a protective role (38), and yet others suggest that it does not play a significant role in the HSV-1 infection process (2, 6). The diversity of findings reported in the literature may reflect the various combinations of mouse strains and virus strains used in the analyses and the different methods used to assess the protective role of IL-2 in the process of HSV-1 infection.
Clarification of the role of IL-2 in the protective responses of the host to ocular HSV-1 infection is necessary both for an improved understanding of the natural history and pathology of this disease and for an accurate assessment of the therapeutic potential of exogenous administration of IL-2. The studies presented here utilized a recombinant HSV-1 virus constructed so that it expresses the IL-2 gene (HSV-IL-2) in an attempt to determine directly the role, if any, that IL-2 plays in ocular HSV-1 infection. Using this approach, we have shown that (i) IL-2 appears to protect against ocular HSV infection, as HSV-IL-2 proved to be less virulent than either the wild-type virus or its marker-rescued virus (the survival of mice coinfected with the parental virus and HSV-IL-2 was higher than that of mice infected with the parental virus alone, and depletion of IL-2 resulted in increased virulence of HSV-IL-2) and (ii) the ability of IL-2 to protect against ocular HSV-1 infection appears to be related to the activity of both the CD4+ and CD8+ T-cell populations, as depletion of either type of T cell resulted in a higher mortality rate upon HSV-IL-2 infection.
MATERIALS AND METHODS
Viruses and cells.
The growth and preparation of triply plaque-purified wild-type HSV-1 strain McKrae and dLAT2903 HSV-1 have been described previously (30). Rabbit skin (RS) cells were grown in Eagle's minimal essential medium supplemented with 5% fetal calf serum. L929 cells were grown in RPMI 1640 supplemented with 10% fetal calf serum.
Mice.
Inbred BALB/c mice (The Jackson Laboratory, Bar Harbor, Maine) were used. All mice used were between 5 and 8 weeks of age.
Construction of IL-2 plasmid.
A plasmid containing the murine IL-2 gene was digested with PstI and BglI (ATCC clone 37553). This insert contained the complete 169-amino-acid-coding region of the IL-2 gene plus 7 and 232 bp of noncoding sequence in its 5′ and 3′ regions, respectively. To construct the IL-2 gene-containing plasmid pLAT1.6, which was used to construct dLAT2903, parental virus (15, 30) was digested with BamHI. pLAT1.6 contained 880 bp upstream of the BamHI site and 2,989 bp downstream of the BamHI site. The IL-2 insert was ligated into the BamHI site of pLAT1.6, and the resulting plasmid was designated pLAT-IL-2. This plasmid contains the 746-bp IL-2 gene bounded by 880- and 2,989-bp LAT fragments.
Generation of HSV-IL-2.
The complete IL-2 gene including its polyadenylation site was inserted just downstream of the LAT promoter by homologous recombination as described previously (15). Briefly, pLAT-IL-2 was cotransfected with infectious dLAT2903 DNA by the calcium phosphate method. Viruses from the cotransfection were plated, and isolated plaques were picked and then screened for insertion of the IL-2 gene by restriction digestion and Southern blot analysis. A single plaque containing the IL-2 gene was plaque-purified eight times and then reanalyzed by restriction digestion and Southern blot analysis to ensure that the IL-2 DNA was present in the LAT region. A single plaque meeting this criterion was chosen for purification and designated HSV-IL-2. HSV-IL-2 therefore transcribes mouse IL-2 driven by the LAT promoter. It should be noted that LAT RNA is not transcribed by this virus, and consequently the parental LAT− dLAT2903 was used as a control in the studies described here. HSV-IL-2 and dLAT2903 are identical except for the insertion of the IL-2 gene in place of the LAT region deleted in dLAT2903. Thus, HSV-IL-2 contains exactly the same LAT nucleotides as dLAT2903.
Virus replication in tissue culture.
RS cell monolayers at 70 to 80% confluency were infected with HSV-IL-2 at 0.01, 1, and 10 PFU/cell. Virus was harvested at the indicated time points by two cycles of freeze-thawing of the cell monolayers with medium. Virus titers were determined by standard plaque assays on RS cells as we have described previously (14). In some experiments, 5 μg of anti-IL-2 polyclonal antibody (R&D Systems) was incubated with 106 PFU of HSV-IL-2 for 1 h at 37°C. Virus (and anti-IL-2) was added to RS cells at an MOI of 1 in six-well microtiter plates, and the plates were incubated at 37°C for 24, 48, or 72 h. Virus titers were determined by standard plaque assays on RS cells as above.
Ocular challenge.
Mice were challenged ocularly with 2 × 107, 2 × 106, 2 × 105, or 2 × 104 PFU of wild-type HSV-1 strain McKrae, dLAT2903, or HSV-IL-2 per eye in 5 μl of tissue culture medium using an eye drop technique without corneal scarification (14). In some experiments, mice were challenged ocularly with a mixture of 2 × 105 PFU of HSV-IL-2 and 2 × 105 PFU of dLAT2903 per eye in 5 μl of tissue culture medium.
Titration of virus in tears.
Tear films were collected from both eyes of five mice per group at various times as we have described previously (11). Each swab was placed in tissue culture medium (0.5 ml), and the amount of virus in the medium was determined by a standard plaque assay on RS cells.
Titer of infectious virus in the whole eye, brain, and TG.
On day 3, 5, or 7 postinfection, mice were euthanized, and the trigeminal ganglia (TG), eyes, and brains were isolated and homogenized individually as we have described previously (11). The cell debris was removed by centrifugation at 3,000 rpm for 10 min with a Beckman TA10 rotor. The virus titer in the supernatant was then measured by using a plaque assay on RS cells as we have described previously (16).
Depletion of CD4+ or CD8+ T cells.
Each mouse received an intraperitoneal injection of 100 μg of purified GK1.5 (anti-L3T4 [CD4+]), 2.43 (anti-Lyt-2 [CD8+]), or both monoclonal antibodies (National Cell Culture Center, Minneapolis, Minn.) in 100 μl of phosphate-buffered saline (PBS) 96 and 24 h before ocular challenge, as we have described previously (12). The injections were repeated 24 and 96 h after ocular challenge. The efficiency of CD4+and CD8+ T-cell depletion was monitored by fluorescence-activated cell sorting analysis of spleen cells 24 h after the second depletion.
Depletion of IL-2.
Each mouse received an intravenous injection of 2 mg of purified anti-IL-2 monoclonal antibody (clone S4B6) (National Cell Culture Center) in 50 μl of PBS 96 and 24 h before ocular challenge, as we have described previously (12). The injections were repeated 24 and 96 h after ocular challenge.
Lymphocyte proliferation response.
The spleens were removed from challenged mice, and single-cell suspensions were prepared. The cells were stimulated in vitro with 5 PFU of UV-inactivated wild-type HSV-1 strain McKrae per cell for 24 h, and on day 2, 1 μCi of [3H]thymidine was added to 2 × 105 lymphocytes, with the incorporation of [3H]thymidine being evaluated 24 h later, as described previously (14). Controls included unstimulated lymphocytes from challenged mice and lymphocytes stimulated with 25 μg of lipopolysaccharide (Sigma Chemical) per 2 × 105 lymphocytes.
IL-2 assay.
RS or L929 cell monolayers at 70 to 80% confluency were infected with HSV-IL-2 at 10 PFU/cell. Infected cells were frozen at the time points indicated, and the presence of IL-2 in the cell-free culture supernatants was assayed in triplicate by using IL-2-specific enzyme-linked immunosorbent assays (ELISAs) (BD, San Diego, Calif.). The concentrations of IL-2 in the supernatants were estimated by comparing the optical densities of the unknowns to those of the IL-2 standard and are presented as mean picograms per milliliter ± standard error of the mean.
Statistical analysis.
Protective parameters were analyzed by Student's t test and Fisher's exact test using Instat (GraphPad, San Diego, Calif.). Results were considered statistically significant when the P value was <0.05.
RESULTS
Structure of HSV-IL-2.
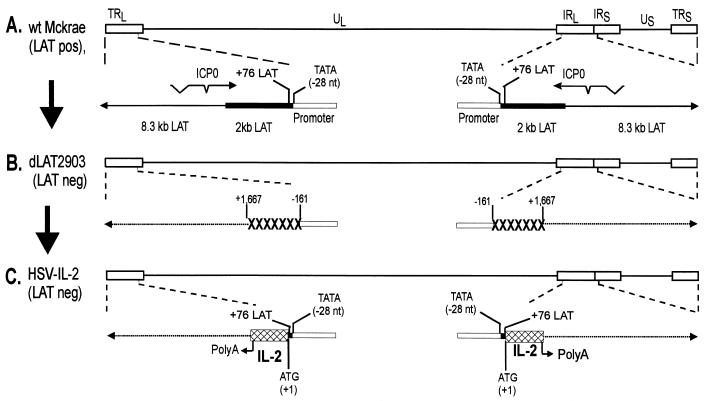
To examine the potential role of IL-2 in protection against ocular HSV-1 infection, we constructed a recombinant version of HSV-1 that expresses IL-2 by using the strategy described in Materials and Methods. The dLAT2903 virus, which was derived from the wild-type HSV-1 strain McKrae, was used as the parental virus (30). The genomic structures of the wild-type HSV-1 strain McKrae and the dLAT2903 virus are shown schematically in Fig. 1A and 1B. The location of the TATA box within the LAT promoter is indicated as −28 (40). In the dLA2903 virus, the LAT promoter and the first 1,667 nucleotides of the LAT transcript have been deleted, and therefore this virus does not make any LAT transcript (30). We established previously that dLAT2903 is identical to wild-type HSV-1 strain McKrae in terms of replication in tissue culture, as well as in eyes, TG, and brain after ocular infection, and that the two viruses display similar virulence after ocular infection (30).
FIG. 1.
Construction and structure of HSV-IL-2. (A) This schematic shows the wild-type (wt) HSV-1 strain McKrae genome in the prototypic orientation. TRL and IRL represent the terminal and internal (or inverted) long repeats, respectively, and TRS and IRS represent the terminal and internal (or inverted) short repeats, respectively. UL and US represent the long and short unique regions, respectively. The solid rectangle represents the very stable 2-kb LAT. The arrow at +1 indicates the start site for LAT transcription. (B) dLAT2903 has a deletion of LAT nucleotides −161 to +1667 relative to the start of LAT transcription in both copies of LAT. The dashed lines indicate no synthesis of LAT RNA. (C) HSV-IL-2 was constructed by homologous recombination between dLAT2903 DNA and a plasmid containing the complete LAT promoter and the entire structural IL-2 gene (including its 3′ polyadenylation signal) bounded by 880- and 2,989-bp LAT fragments as described in Materials and Methods.
HSV-IL-2 was derived from dLAT2903 by the insertion of the IL-2 gene under the control of the LAT promoter (Fig. 1C) using the same method that was used to create dLAT2903 except that HSV-IL-2 includes two copies of the IL-2 gene. As confirmed by restriction enzyme analysis and partial DNA sequencing, this construct contained the entire sequence of the IL-2 gene. The HSV-IL-2 gene includes a noncoding region of 7 nucleotides upstream of the first ATG, followed by the complete coding region of IL-2 (507 nucleotides). A noncoding region (232 nucleotides) is included downstream of the 3′ IL-2 termination codon that includes its polyadenylation region. Except for restoration of the LAT promoter, HSV-IL-2 has the same deletion as dLAT2903.
Expression of IL-2 by HSV-IL-2 in tissue culture.
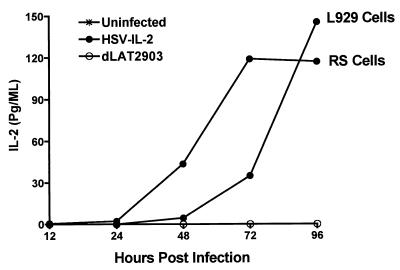
Confluent monolayers of RS cells and mouse L929 cells were infected with HSV-IL-2, dLAT2903, or wild-type HSV-1 strain McKrae at a multiplicity of 1 PFU/cell for periods ranging from 0 to 96 h. Medium was collected at the indicated times, and the presence of IL-2 protein was determined by using anti-IL-2 monoclonal antibody in an ELISA, as described in Materials and Methods. Prior to infection, neither the RS nor the L929 cells expressed detectable levels of IL-2 in the supernatant (Fig. 2). IL-2 was also not detectable in supernatants obtained from RS or L929 cells infected with either dLAT2903 (Fig. 2) or wild-type HSV-1 strain McKrae (not shown).
FIG. 2.
Kinetics of expression of IL-2 by HSV-IL-2 in tissue culture. Subconfluent RS or L929 cell monolayers were infected with 1 PFU of HSV-IL-2 or dLAT2903 per cell as described in Materials and Methods. Supernatants were harvested from cell cultures at the indicated times postinfection, and the amount of IL-2 in the medium was determined by ELISA as described in Materials and Methods.
After infection with HSV-IL-2, however, significant levels of IL-2 were detected in the RS cell cultures, with the maximal level being attained 72 h after infection and sustained through the end of the assay period (96 h) (Fig. 2). Similarly, significant levels of IL-2 were detected in the supernatants of the HSV-IL-2-infected L929 cells. The kinetics of expression differed from those of the HSV-IL-2-infected RS cells, however, with the levels of IL-2 being significantly lower at 72 h after infection (Fig. 2) but exhibiting a dramatic increase (≈5-fold) by 96 h, so that the IL-2 levels in the supernatants of the HSV-IL-2-infected L929 cell cultures were higher than those of the HSV-IL-2-infected RS cells at this time. These results suggest that the recombinant HSV-1-IL-2 is capable of expressing IL-2 for at least 4 days postinfection.
Effect of ocular infection with HSV-IL-2 on lymphocyte proliferation.
It has been reported that exogenous application of IL-2 can amplify the proliferation of T lymphocytes in vivo (26). To determine if IL-2 expressed by HSV-IL-2 has biological activity in vivo, we investigated the ability of HSV-IL-2 to increase the lymphoproliferative responses of mice infected with HSV-IL-2 in comparison with the responses of mice infected with parental virus. Mice were challenged ocularly, and their spleens were harvested on day 3 after challenge. The proliferative response of the splenic lymphocytes in single-cell suspensions was assessed as described in Materials and Methods.
As expected, incorporation of [3H]thymidine was not observed in the absence of in vitro priming with UV-inactivated HSV-1 of the splenic lymphocytes of any of the groups of mice (Table 1). The proliferative response to stimulation with UV-inactivated HSV-1 was significantly higher in the spleen cell preparations from mice infected with HSV-IL-2 than in those from mice infected with dLAT2903 (P = 0.0001, Student's t test) (Table 1). Similarly, a higher proliferative response to lipopolysaccharide was observed in the mice infected with HSV-IL-2 than in those infected with dLAT2903. Therefore, our results suggest that the HSV-IL-2 virus induced a stronger immune response than the parental dLAT2903 virus and that the IL-2 expressed by the HSV-IL-2 recombinant virus is biologically active in vivo.
TABLE 1.
Lymphocyte proliferative response of mice infected with HSV-IL-2a
| Stimulation | Mean [3H]thymidine incorporation (cpm) ± SEM
|
P (Student's t test) | |
|---|---|---|---|
| HSV-IL-2 | dLAT2903 | ||
| Virus | 5,673 ± 469 | 2,732 ± 176 | 0.0001 |
| Lipopolysaccharide | 62,287 ± 3,851 | 45,883 ± 1,772 | 0.0054 |
| None | 43 ± 13 | 80 ± 32 | 0.89 |
Mice were inoculated ocularly with HSV-IL-2 or dLAT2903, and 3 days later the spleens were removed from the euthanized mice. The splenocyte preparations were primed in vitro for 24 h, and then the cells were cultured with [3H]thymidine for 24 h as described in Materials and Methods. Each value represents the mean for eight replicates from two experiments.
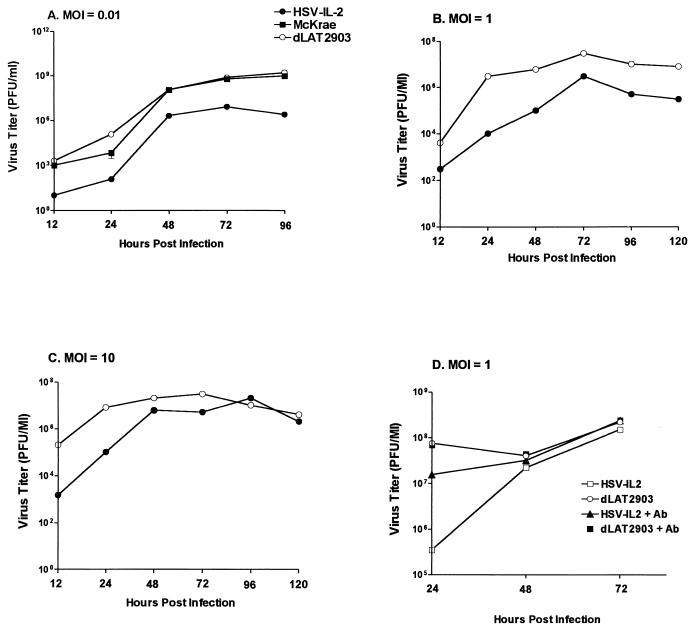
Replication of HSV-IL-2 in tissue culture.
To determine whether the recombinant HSV-IL-2 replicated in tissue culture as efficiently as the wild-type HSV-1 strain McKrae and parental dLAT2903 viruses, RS cells were infected in triplicate with 0.01, 1, or 10 PFU of HSV-IL-2, dLAT2903, or wild-type HSV strain McKrae per cell. The cell monolayers were freeze-thawed at the indicated time points, and the yield of infectious virus was quantitated by using a plaque assay as described in Materials and Methods. At an MOI of 0.01 per cell, the HSV-IL-2-infected cells exhibited significantly lower virus yield than the parental virus-infected cells (P < 0.05, Student's t test) (Fig. 3A), with the parental virus and wild-type HSV-1 strain McKrae exhibiting similar titers (P > 0.05, Student's t test). At an MOI of 1 (Fig. 3B) or 10 (Fig. 3C), the yield of virus was time dependent, with the yield of HSV-IL-2 being lower than the yield of dLAT2903 at 12 and 24 h postinfection (P < 0.05, Student's t test). By 48 h postinfection, however, the titers of HSV-IL-2 had attained levels similar to those of the dLAT2903 viruses in RS cells.
FIG. 3.
HSV-IL-2 replication in tissue culture. Subconfluent RS cell monolayers were infected with 0.01, 1, or 10 PFU of HSV-IL-2, parental dLAT2903, or wild-type McKrae virus per cell with or without incubation with anti-IL-2 polyclonal antibody, as described in Materials and Methods. Total virus was harvested at the indicated times postinfection by two cycles of freeze-thawing. The amount of virus at each time for each virus was determined by standard plaque assays on RS cells.
The above data suggest that in tissue culture, replication of HSV-IL-2 was decreased compared to that of dLAT2903 and the wild type. In particular, at an MOI of 1, at 24 h postinfection, replication of HSV-IL-2 was less than 100-fold that of dLAT2903. To determine if the decreased replication of HSV-IL-2 might be related to expression of IL-2, we examined the affect of anti-IL-2 polyclonal antibody on HSV-IL-2 replication. As described in Materials and Methods, the virus was mixed with 5 μg of anti-IL-2 polyclonal antibody, and then cells were infected at an MOI of 1 with the virus and antibody (Fig. 3D). In the presence of anti-IL-2 antibody, replication of HSV-IL-2 increased significantly and was similar to that of dLAT2903 with or without anti-IL-2 antibody. This finding suggests that expression of IL-2 by HSV-IL-2 decreased virus replication in tissue culture.
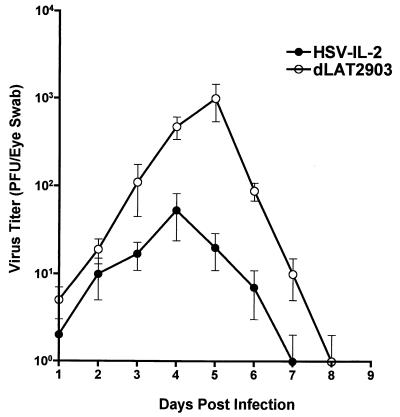
Virus titers in mouse tears after ocular infection.
BALB/c mice were infected ocularly with 2 × 105 PFU of either HSV-IL-2 or dLAT2903 per eye as described in Materials and Methods. From day 1 to day 9 postinfection, tear films were collected from five mice (10 eyes) per group, and the amount of virus was assessed by plaque assays on RS cells (Fig. 4). The peak titer of HSV-IL-2 in the tears (≈102 PFU per eye) was lower than the peak titer of dLAT2903 virus (≈103 PFU per eye), with the difference in titer being highly significant (P < 0.001, Student's t test).
FIG. 4.
HSV-IL-2 replication in mouse tears after ocular infection. BALB/c mice were infected with 2 × 105 PFU of HSV-IL-2 or dLAT2903 per eye. Tear films were collected daily on days 1 to 9, and virus titers were determined by standard plaque assays. Each point represents the mean titer ± standard error of the mean in tears from 10 eyes.
To determine if the strain of mouse affects the behavior of HSV-IL-2, C57BL/6 mice were infected with a 10-fold-higher challenge dose (2 × 106 PFU/eye) of HSV-IL-2, dLAT2903, or wild-type HSV-1 strain McKrae (not shown). As in BALB/c mice, the titers of HSV-IL-2 were lower than the titers of dLAT2903 or strain McKrae virus (data not shown). The titers of dLAT2903 virus and wild-type HSV-1 strain McKrae were similar.
Virus replication in whole eyes, TG, and brains.
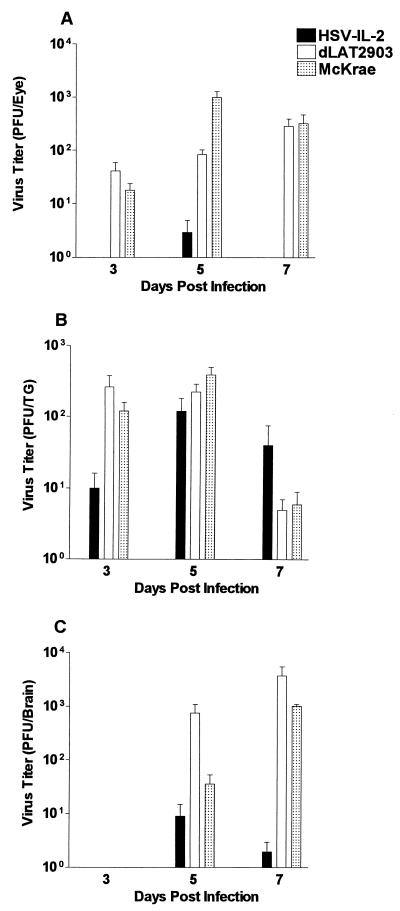
BALB/c mice from two separate experiments were infected ocularly with HSV-IL-2, dLAT2903, or wild-type HSV-1 strain McKrae as described in Materials and Methods. On days 3, 5, and 7 postinfection, seven mice per group (four mice from experiment 1 and three mice from experiment 2) were sacrificed, and the eyes, TG, and brains were harvested for analysis of the titer of infectious virus as described in Materials and Methods. The data from both experiments were combined and are presented in Fig. 5.
FIG. 5.
Virus titers in whole eyes, TG, and brain after ocular infection. BALB/c mice were infected ocularly with 2 × 105 PFU of HSV-IL-2, dLAT2903, or wild-type HSV-1 strain McKrae per eye as described in Materials and Methods. Mice were euthanized on the indicated days. Eyes (A), TG (B), and brain (C) were removed and homogenized, and virus titers were determined as described in Materials and Methods. A total of nine mice per time point were analyzed from two separate experiments. Each point represents the mean titer ± standard error of the mean for extracts of 18 eyes, 18 TG, or 9 brains.
Analysis of whole eyes indicated that the HSV-IL-2 virus was not detectable 3 days after ocular infection of BALB/c mice (Fig. 5A). There was a small amount of HSV-IL-2 on day 5, followed by complete virus clearance by day 7 (Fig. 5A). In contrast, both dLAT2903 and wild-type HSV-1 strain McKrae were detectable at day 3 postinfection and increased to a peak level on days 5 and 7. The differences in the virus titers of HSV-IL-2 and those of the parental or McKrae viruses in whole eyes were highly significant (P < 0.001, Student's t test) (Fig. 5A).
Although analysis of the TG at day 3 postinfection similarly indicated that the average amount of HSV-IL-2 was lower than that of either dLAT2903 or the wild-type HSV strain McKrae (P < 0.001, Student's t test) (Fig. 5B), HSV-IL-2 was detectable at day 3, and the titers increased from day 3 to day 5, attaining levels similar to those in the control groups (P > 0.05, Student's t test) (Fig. 5B). Between days 5 and 7 postinfection, the virus titers in the TG of all three groups exhibited a decline, but the rate of decline of the virus titer in the TG of HSV-IL-2-infected mice was slower, and by day 5, the virus titer in these mice was higher than the virus titers in control mice (P < 0.01, Student's t test) (Fig. 5B).
Analysis of the brain indicated a pattern of replication of HSV-IL-2 similar to that in the whole eyes, with HSV-IL-2 being undetectable at day 3 postinfection (Fig. 5C). By day 5 postinfection, virus was detectable in the brains, with the average virus titer in the brains of HSV-IL-2-infected mice being significantly lower than the average titers in the brains of dLAT2903- or wild-type HSV-1 strain McKrae-infected mice (P < 0.001, Student's t test) (Fig. 5C). These titers increased through day 7 postinfection in all groups of mice except the HSV-IL-2-infected mice, which showed a decline in virus titer (P < 0.0001, Student's t test) (Fig. 5C). Taken together, our results suggest that HSV-IL-2 replicated to a lower titer in the eyes and brains of infected mice than did the parental dLAT2903 and wild-type HSV-1 strain McKrae viruses. Interestingly, HSV-IL-2 replicated to higher titers in the TG.
Virulence of HSV-IL-2 in BALB/c mice.
To determine the differences in virulence between the HSV-IL-2 and parental dLAT2903 or McKrae virus, groups of 5 to 60 BALB/c mice were infected ocularly with 10-fold serial dilutions of either HSV-IL-2, wild-type HSV-1 strain McKrae, or dLAT2903 as described in Materials and Methods. None of the mice survived infection with the dLAT2903 virus or wild-type HSV-1 strain McKrae virus at doses greater than 2 × 105 PFU/eye (Table 2). In contrast, most of the mice infected with HSV-IL-2 survived ocular infection, even at the maximal dose of 2 × 107 PFU/eye (Table 2). These differences in protection in mice infected with HSV-IL-2 and those infected with dLAT2903 or McKrae were highly significant (P < 0.001, Fisher's exact test). Thus, our results suggest that the IL-2-expressing HSV-1 is less virulent than its parental dLAT2903 virus.
TABLE 2.
Survival of BALB/c mice following ocular challenge with various amounts of HSV-IL-2a
| Virus | No. of mice surviving/total (% surviving) after challenge with:
|
|||
|---|---|---|---|---|
| 2 × 104 PFU/eye | 2 × 105 PFU/eyeb | 2 × 106 PFU/eye | 2 × 107 PFU/eye | |
| HSV-IL-2 | 5/5 (100) | 54/60 (90) | 13/15 (87)c | 10/10 (100) |
| McKrae | 0/5 (0) | 4/45 (9) | 0/5 (0) | NDd |
| dLAT2903 | 0/5 (0) | 6/45 (13) | 0/5 (0) | ND |
| HSV-IL-2Re | ND | 0/10 (0) | ND | ND |
Mice were inoculated ocularly with various amounts of each virus, and survival was determined as described in Materials and Methods.
Data from three separate experiments were combined.
Data from two separate experiments were combined.
ND, not done.
Rescued virus.
The appropriate interpretation of the results generated with HSV-IL-2 required confirmation that the differences observed could be attributed to its expression of IL-2 rather than altered behavior due to introduction of the gene. To address this question, a rescued virus was generated by cotransfection and homologous recombination of DNA from the infectious HSV-IL-2 with the original pLAT1.6 plasmid, as described in Materials and Methods. This rescued virus exhibited a pattern of neurovirulence similar to that of the dLAT2903 virus and wild-type HSV-1 strain McKrae, in that 10 of 10 mice infected with 2 × 105 PFU of this virus per eye died from infection (Table 2). These results provide evidence that it is the production of IL-2 by the HSV-IL-2 itself rather than a defect in the recombinant virus that mediates the significantly lower mortality of HSV-IL-2-infected mice.
Effects of coinfection of BALB/c mice with HSV-IL-2 and dLAT2903.
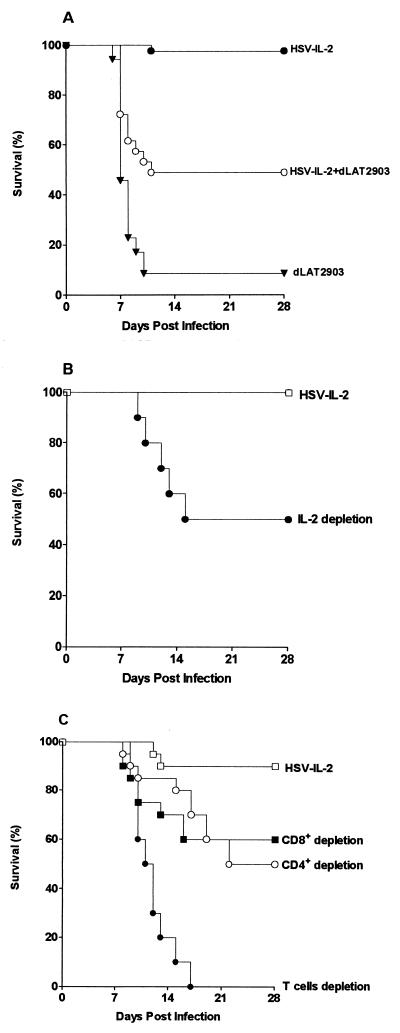
Similarly, if the production of IL-2 by HSV-IL-2 protects the infected mice, it would be predicted that coinfection of the mice with HSV-IL-2 would protect the mice from the lethal effects of the parental dLAT2903 virus. To test this hypothesis, groups of 20 BALB/c mice from two separate experiments were challenged ocularly with 2 × 105 PFU of HSV-IL-2 alone, dLAT2903 alone, or both HSV-IL-2 and dLAT2903 per eye. The data from two separate experiments were combined. As found previously, a high percentage of mice (18 of 20) survived ocular infection with HSV-IL-2, whereas only 1 of 20 mice (5%) survived ocular infection with dLAT2903 (Fig. 6A). In contrast, 10 of 20 mice (50%) coinfected with both viruses survived the lethal challenge (P = 0.003 and P = 0.01 versus dLAT2903 and HSV-IL-2, respectively) even though these mice received the amount of dLAT2903 that had killed 19 of 20 mice.
FIG. 6.
Survival following infection with HSV-IL-2. BALB/c mice were infected with 2 × 105 PFU/eye. Survival was monitored for 28 days after ocular challenge. (A) Survival of mice coinfected with HSV-IL-2, HSV-IL-2 and dLAT2903, or dLAT2903. (B) Survival of IL-2-depleted mice after ocular infection with HSV-IL-2. (C) Survival of CD4+-depleted mice, CD8+-depleted mice, and mice from which both CD4+ and CD8+ T cells were depleted after ocular infection with HSV-IL-2.
These results suggest that the IL-2 produced by HSV-IL-2 either directly or indirectly protected mice against infection with virulent dLAT2903. Combined with data indicating that the marker-rescued virus had wild-type virulence, these results strongly support the contention that HSV-IL-2 does not show reduced virulence because of a defect, but rather that the IL-2 expressed by HSV-IL-2 can provide protection against HSV-1 infection.
Effect of IL-2 depletion on survival of mice infected with HSV-IL-2.
To determine if IL-2 depletion would increase the neurovirulence of HSV-IL-2, mice were depleted of IL-2 both prior to and after infection as described in Materials and Methods. As above, all 10 of the undepleted control mice survived ocular challenge with 2 × 105 PFU of HSV-IL-2 per eye (Fig. 6B). In contrast, only 5 of 10 (50%) of the IL-2-depleted mice survived the challenge with HSV-IL-2 (P = 0.03, Fisher's exact test). These results suggest that the IL-2 expressed by HSV-IL-2 played a role in protecting naive mice against death following HSV-IL-2 infection.
Survival following HSV-IL-2 challenge of T-cell-depleted mice.
As IL-2 has a profound effect on the T-cell immune response, 20 naive mice per group were depleted of CD4+, CD8+, or both CD4+ and CD8+ T cells, as described in Materials and Methods. In the undepleted control group, 95% (19 of 20) of the mice survived ocular challenge with 2 × 105 PFU of HSV-IL-2 per eye (Fig. 6C). In contrast, only 10 of 20 (50%) of the CD4+-depleted mice and 12 of 20 (60%) of the CD8+-depleted mice survived the challenge with HSV-IL-2 (P < 0.01 versus control mice; Fisher's exact test), and none of the mice depleted of both sets of T cells survived (0 of 20) (Fig. 6C; P < 0.003 versus CD4+ or CD8+ T-cell depleted). These results suggest that both CD4+ and CD8+ T cells play an important role in protecting naive mice against ocular infection with HSV-IL-2 and that their protective effects appeared to be additive.
Virus titers in mice depleted of T cells.
The studies of T-cell-depleted mice suggested that the T cells play a critical role in the survival of HSV-IL-2-infected mice. Therefore, the effects of the absence of CD4+ T cells, CD8+ T cells, or both on virus replication in the eyes, TG, and brains of HSV-IL-2-infected mice were assessed. BALB/c mice were depleted of their T-cell populations as described above and then infected ocularly. Virus titers in the eyes, TG, and brain of T-cell-depleted HSV-IL-2-infected mice were compared to those in undepleted mice infected with either HSV-IL-2 or dLA2903. On days 3, 5, and 7, three mice per group were sacrificed, and the eyes, TG, and brains were harvested for analysis of infectious virus as described in Materials and Methods.
Virus was undetectable in the brains of any of the mice on day 3 under any of the infection conditions and irrespective of the T-cell status of the mice (Table 3). On day 5, the average yields of HSV-IL-2 from the brain were similar for control mice and mice depleted of CD4+ or CD8+ T cells (P > 0.05, Student's t test). In contrast, the HSV-IL-2-infected mice depleted of both types of T cells had virus titers higher than those of the undepleted HSV-IL-2-infected mice (P < 0.05, Student's t test) (Table 3). By day 7 postinfection, the virus titers for all depleted groups were increased compared to those in the undepleted HSV-IL-2-infected mice (Table 3). The HSV-IL-2 titers in the brains of mice depleted for both T cell types on day 7 were not significantly different from that of the dLAT2903-infected mice (P = 0.14, Student's t test). This lack of significance may be due to the small number of mice, and additional experiments will be needed to determine if depletion of both T-cell types fully restores replication of HSV-IL-2 in the brain to parental levels.
TABLE 3.
Virus titers in whole eyes, TG, and brains of T-cell-depleted mice after ocular infectiona
| Organ | Mean titer (PFU) ± SEM
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HSV-IL-2
|
dLAT2903
|
||||||||||||||
| CD4 depleted
|
CD8 depleted
|
CD4 + CD8 depleted
|
Undepleted control
|
||||||||||||
| Day 3 | Day 5 | Day 7 | Day 3 | Day 5 | Day 7 | Day 3 | Day 5 | Day 7 | Day 3 | Day 5 | Day 7 | Day 3 | Day 5 | Day 7 | |
| Brain | 0 | 36 ± 32 | 160 ± 29 | 0 | 3 ± 8 | 433 ± 185 | 0 | 287 ± 167 | 406 ± 297 | 0 | 21 ± 12 | 5 ± 1 | 0 | 750 ± 326 | 3,717 ± 1,821 |
| Eye | 13 ± 13 | 18 ± 6 | 565 ± 352 | 23 ± 23 | 55 ± 19 | 267 ± 120 | 147 ± 101 | 253 ± 215 | 464 ± 300 | 1 ± 1 | 7 ± 5 | 1 ± 1 | 41 ± 18 | 84 ± 19 | 282 ± 109 |
| TG | 47 ± 33 | 45 ± 29 | 6 ± 3 | 18 ± 17 | 62 ± 30 | 12 ± 8 | 27 ± 16 | 283 ± 122 | 93 ± 83 | 20 ± 13 | 10 ± 2 | 8 ± 6 | 260 ± 115 | 226 ± 62 | 5 ± 2 |
BALB/c mice were depleted of CD4+ T cells, CD8+ T cells, or both CD4+ and CD8+ T cells as described in Materials and Methods. Depleted mice were ocularly infected with 2 × 105 PFU of HSV-IL-2 per eye. Control undepleted mice were similarly infected with HSV-IL-2 or dLAT2903 as described in Materials and Methods. Mice were euthanized on the indicated days, eyes, brains, and TG were removed and homogenized, and virus titers were determined as described in Materials and Methods. Each value represents the mean PFU titer of extracts from six eyes, six TG, or three brains.
The yield of virus from the eyes of the control mice infected with HSV-IL-2, which were very low at day 3 postinfection, exhibited a sharp increase by day 5, followed by a rapid decline through day 7 (Table 3). The titers of dLAT2903 virus increased from day 3 to day 5 and stayed at this level until day 7. The virus titers in the eyes of CD4+-depleted mice, CD8+-depleted mice, and mice that had been depleted of both T-cell types increased from day 3 to day 7 postinfection and attained levels similar to those in dLAT2903-infected mice (P > 0.05, Student's t test) (Table 3).
The average yield of HSV-IL-2 in the TG did not differ between the control mice and mice depleted of CD4+ T cells, CD8+ T cells, or both CD4+ and CD8+ T cells (P > 0.05, Student's t test) (Table 3). As described above, the dLAT2903-infected mice had higher titers of virus in the TG than did control mice infected with HSV-IL-2 on day 3 postinfection (P < 0.05, Student's t test) (Table 3). The virus yield from the TG of the dLAT2903- and HSV-IL-2-infected control groups remained unaltered between days 3 and 5. Similarly, the virus yield from the TG of the HSV-IL-2-infected, CD4+-depleted mice remained unaltered during this time period. In contrast, HSV-IL-2 virus yields increased in the TG of mice in which the CD8+ T cells had been depleted (either CD8+ alone or CD4+ and CD8+), so that these mice yielded levels of virus similar to those of mice infected with parental virus at day 5 postinfection. A decline in virus yield in the TG from all groups of mice was observed from day 5 to day 7 (Table 3).
These results suggest that HSV-IL-2 replicated to a higher titer in the eyes, TG, and brains of mice that were depleted of either CD4+ or CD8+ T cells then in HSV-IL-2-infected mice with an intact T-cell system. This increase in virus titers in the eyes, TG, and brains of the T-cell-depleted mice may have contributed to the increased frequency of death among these mice after infection with HSV-IL-2.
DISCUSSION
The replication of virus subsequent to a primary HSV-1 infection of the eye can cause eye disease (17). Both the duration of the infection and the titer of virus in the eye affect the severity of eye disease (13). Therefore, therapeutic strategies that decrease the virus load in the eye and accelerate virus clearance should reduce the severity of eye disease in infected individuals. Our previous results have suggested that endogenous IL-2 can control acute ocular HSV-1 infection in vivo (11, 18). However, the immunotherapeutic potential of the exogenous application of IL-2 in the control of ocular HSV-1 replication has not been established unambiguously. To more clearly define the relationship between exogenous application of IL-2 and protection against ocular HSV-1 infection, we constructed a recombinant HSV-1 that expresses two copies of the IL-2 gene, both of which are under the control of the LAT promoter. The LAT promoter is the only viral promoter that sustains high levels of transcription during latent as well as primary infection (30). We designed this strategy to overcome the problems inherent in interpreting the effects of administration of IL-2, such as the need for repeated injections of recombinant IL-2 (31, 35) and the temporary expression of IL-2 provided by adenoviruses (1).
We first confirmed that cells infected with HSV-IL-2 secreted large quantities of IL-2 in tissue culture and that, as anticipated under conditions of elevated levels of circulating IL-2 (26), the splenocytes of HSV-IL-2-infected mice have a higher proliferative response than those of mice infected with wild-type virus. At a higher MOI, the replication of HSV-IL-2 was confirmed to be comparable to that of the parental virus in vitro, although, at a lower MOI, the replication rates of HSV-IL-2 were significantly lower than those of the parental virus. We further showed that anti-IL-2 antibody increased replication of HSV-IL-2 in tissue culture. This strongly suggests that the expressed IL-2 somehow decreased virus replication, presumably via some direct effect on the cells in the absence of any additional immune components.
After ocular infection, we found that the titers of virus in the tears were lower in HSV-IL-2-infected than parental virus-infected mice. This finding extends our previously published results and further supports the concept that there is a correlation between IL-2 levels and reduced viral replication in the eye (13, 17). Since the expressed IL-2 also appeared to decrease viral replication in tissue culture, it is possible that some of the decreased replication in the eye is due to direct effects of IL-2 on the infected cells in the eye and not simply due to an immune cell response. The literature includes several other reports that support this concept, including the finding that administration of recombinant IL-2 to hepatitis B virus transgenic mice reduced hepatitis B virus mRNA expression by up to 90% (19). Furthermore, in vivo application of recombinant IL-2 has been reported to decrease the titers of virus after murine cytomegalovirus infection in BALB/c mice (31).
Infection with HSV-IL-2 was not lethal to mice, even at an infectious dose of 2 × 107 PFU/eye. In a similar study, infection of athymic nude mice, which normally succumb to virus infection, with recombinant vaccinia virus expressing IL-2 resulted in protection against death (21). Furthermore, coinfection of mice with a mixture of HSV-IL-2 and a lethal dose of dLAT2903 protected 50% of the mice from death, with only 5% of mice surviving infection with an equivalent dose of dLAT2903 alone. Thus, our results suggest that IL-2 protects against HSV-1 infection. Taken together with the failure of the marker-rescued virus to exhibit a similar protective response, these results suggest that the decreased mortality observed in association with HSV-IL-2 infection can be attributed to recombinant expression of the IL-2.
It is well established that IL-2 acts as both a paracrine and an autocrine factor that induces the expansion of antigen-specific T cells as well as enhancing the activity of a number of other cell types, including B cells, NK cells, and LAK cells (36, 37). It has been shown that in vivo administration of IL-2 enhances the activity of both CD4+ and CD8+ T-cell populations (29). Neutrophils (8), monocytes (10), and gamma/delta T cells (25) also exhibit activation, augmented function, or increased survival when exposed to IL-2 (32).
We have shown previously that viral clearance in vaccinated mice is dependent on the level of IL-2 (17, 18). Similarly, in this study we have shown that expression of IL-2 by HSV-1 attenuated virus replication, reducing the virus yield both in vitro and in vivo. In a similar type of study, coexpression of IL-2 by recombinant respiratory syncytial virus was associated with attenuation of virus growth in vivo (4). Furthermore, in the presence of anti-IL-2 antibody, replication of HSV-IL-2 was enhanced in vitro. Similarly, in vivo depletion of IL-2 led to more deaths in infected mice. We previously found that a mutant identical to HSV-IL-2 except that it expresses IL-4 instead of IL-2 (HSV-IL-4) also has attenuated virulence (15). Although this may appear paradoxical, since IL-2 and IL-4 are antagonistic cytokines, it appears instead that this is simply a case of both cytokines being able to provide some protection against HSV-1 infection.
Depletion of either CD4+ or CD8+ T cells produced a reduction in the protective effects of HSV-IL-2 infection of approximately 50%. In addition, depleting both subsets of T cells completely abolished the protective effect, resulting in a 100% death rate following HSV-IL-2 infection. This result is in contrast to a previously published study showing that recombinant vaccinia viruses expressing IL-2 or coexpressing IL-2 and the influenza virus hemagglutinin gene completely protected nude mice from lethal infection with vaccinia and influenza A viruses, respectively (21). The discrepancies between our results and those based on the analysis of vaccinia virus vectors in nude mice could be due to differences in the type of virus. For example, other investigators utilizing IL-4 expression by a recombinant vaccinia virus found exacerbation of infection associated with a T-cell-independent process (5), whereas we did not observe any side effects when using a recombinant HSV-1 expressing IL-4 (15). Also, although both IL-2- and IL-4-expressing recombinant HSV-1 viruses kill fewer mice, the protective mechanism produced by expression of these cytokines is assumed to be different.
At various times postinfection in mouse eyes and brains, the titers of HSV-IL-2 were reduced compared to those in mice infected with the parental dLAT2903 or wild-type McKrae virus. Similarly, at early times postinfection in mouse brain, the titers of HSV-IL-2 were lower than or similar to that in control mice. In contrast, at later times postinfection, more HSV-IL-2 than parental dLA2903 virus was detected in the brains. The reason for the increased amount of HSV-IL-2 detected in the brain is not known. However, it is possible that the TG and the brain responded differently to the expressed IL-2 and that this impacted the outcome of virus replication.
The depletion of the CD4+ and CD8+ T-cell populations either alone or in combination was also associated with higher virus titers in the tears, TG, eyes, and brain. When the mice were differentially depleted of CD4+ or CD8+ T cells, the remaining T-cell subtype was sufficient to prevent an increase in virus titers in the TG of the infected mice. In contrast, viral clearance from the eyes or brains of HSV-IL-2-infected mice required both the CD4+ and CD8+ T-cell subtypes.
The roles of CD4+ and CD8+ T cells in the control of acute HSV-1 infection have been reported to differ in the skin and nervous system (24). The protection from lethality observed in HSV-IL-2-infected mice was mediated by both CD4+ and CD8+ T cells. Furthermore, it was associated with reduced virus titers at the site of infection and lower neurotoxicity due to the expression of IL-2, since depletion of IL-2 by anti-IL-2 monoclonal antibody significantly enhanced the pathogenicity of the HSV-IL-2 virus. In this study we found that depletion of either T-cell type led to higher death rates in HSV-IL-2-infected mice. Thus, the protection afforded by HSV expression of IL-2 was T-cell dependent, whereas the protection induced by recombinant vaccinia virus expressing IL-2 is T-cell independent (21).
While protection of the HSV-IL-2-infected mice was associated with both the CD4+ and CD8+ T-cell populations, the protective effects of a recombinant HSV-1 virus that expresses IL-4 have been shown to be associated with CD4+ T-cell activity but not CD8+ T-cell activity (15). These results are in concordance with the reports that T helper (TH)1 cells, which are characterized by the production of IL-2 among other cytokines, enhance both delayed-type hypersensitivity and cellular cytotoxicity, whereas TH2 cells, which are characterized by the production of IL-4 among other cytokines, are involved in antibody-mediated immunity (22, 23, 28, 34). In contrast to the IL-2- and IL-4-expressing recombinant HSV-1 viruses, a recombinant HSV-1 expressing gamma interferon did not induce protection at similar challenge doses, suggesting that the protective TH1 response to HSV-1 is associated with IL-2 rather than gamma interferon (H. Ghiasi, unpublished data).
In summary, our data support the concept that IL-2 plays an important role in HSV-1 protection and suggest that the IL-2-expressing virus is less neurovirulent than its parental virus, protects infected mice through a mechanism involving both CD4+ and CD8+ T cells, and is cleared from the eyes and brain more rapidly than the parental virus.
Acknowledgments
This work was supported by Public Health Service grants EY09224 and EY13615 from the National Eye Institute, by the Discovery Fund for Eye Research, and by the Skirball Program in Molecular Ophthalmology to H.G.
REFERENCES
- 1.Addison, C. L., T. Braciak, R. Ralston, W. J. Muller, J. Gauldie, and F. L. Graham. 1995. Intratumoral injection of an adenovirus expressing interleukin 2 induces regression and immunity in a murine breast cancer model. Proc. Natl. Acad. Sci. USA 92:8522-8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, E. M., J. P. Weir, S. Martin, C. Mercadal, and B. T. Rouse. 1990. Role of coexpression of IL-2 and herpes simplex virus proteins in recombinant vaccinia virus vectors on levels of induced immunity. Viral Immunol. 3:207-215. [DOI] [PubMed] [Google Scholar]
- 3.Babu, J. S., S. Kanangat, and B. T. Rouse. 1995. T cell cytokine mRNA expression during the course of the immunopathologic ocular disease herpetic stromal keratitis. J. Immunol. 154:4822-4829. [PubMed] [Google Scholar]
- 4.Burkreyev, A., S. S. Whitehead, C. Prussin, B. R. Murphy, and P. L. Collins. 2000. Effect of coexpression of interleukin-2 by recombinant respiratory syncytial virus on virus replication, immunogenicity, and production of other cytokines. J. Virol. 74:7151-7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheers, C., M. Janas, A. Ramsay, and I. Ramshaw. 1999. Use of recombinant viruses to deliver cytokines influencing the course of experimental bacterial infection. Immunol. Cell. Biol. 77:324-330. [DOI] [PubMed] [Google Scholar]
- 6.Chun, S., M. Daheshia, N. A. Kuklin, and B. T. Rouse. 1998. Modulation of viral immunoinflammatory responses with cytokine DNA administered by different routes. J. Virol. 72:5545-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson, C. R. 1984. Ocular herpes simplex virus infections. Clin. Dermatol. 2:56-66. [DOI] [PubMed] [Google Scholar]
- 8.Djeu, J. Y., J. H. Liu, S. Wei, H. Rui, C. A. Pearson, W. J. Leonard, and D. K. Blanchard. 1993. Function associated with IL-2 receptor-beta on human neutrophils. Mechanism of activation of antifungal activity against Candida albicans by IL-2. J. Immunol. 150:960-970. [PubMed] [Google Scholar]
- 9.Easty, D. L., C. Shimeld, C. M. Claoue, and M. Menage. 1987. Herpes simplex virus isolation in chronic stromal keratitis: human and laboratory studies. Curr. Eye Res. 6:69-74. [DOI] [PubMed] [Google Scholar]
- 10.Espinoza-Delgado, I., M. C. Bosco, T. Musso, G. L. Gusella, D. L. Longo, and L. Varesio. 1995. Interleukin-2 and human monocyte activation. J. Leukoc. Biol. 57:13-19. [DOI] [PubMed] [Google Scholar]
- 11.Ghiasi, H., S. Bahri, A. B. Nesburn, and S. L. Wechsler. 1995. Protection against herpes simplex virus-induced eye disease after vaccination with seven individually expressed herpes simplex virus 1 glycoproteins. Investig. Ophthalmol. Vis. Sci. 36:1352-1360. [PubMed] [Google Scholar]
- 12.Ghiasi, H., S. Cai, G. C. Perng, A. B. Nesburn, and S. L. Wechsler. 2000. Both CD4+ and CD8+ T cells are involved in protection against HSV-1 induced corneal scarring. Br. J. Ophthalmol. 84:408-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghiasi, H., S. Cai, S. M. Slanina, G. C. Perng, A. B. Nesburn, and S. L. Wechsler. 1999. The role of interleukin (IL)-2 and IL-4 in herpes simplex virus type 1 ocular replication and eye disease. J. Infect. Dis. 179:1086-1093. [DOI] [PubMed] [Google Scholar]
- 14.Ghiasi, H., R. Kaiwar, A. B. Nesburn, S. Slanina, and S. L. Wechsler. 1994. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): comparative protection against lethal challenge in mice. J. Virol. 68:2118-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghiasi, H., Y. Osorio, G. C. Perng, A. B. Nesburn, and S. L. Wechsler. 2001. Recombinant herpes simplex virus type 1 expressing murine interleukin-4 is less virulent than wild-type virus in mice. J. Virol. 75:9029-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghiasi, H., G. C. Pemg, F. M. Hofman, S. Cai, A. B. Nesburn, and S. L. Wechsler. 1999. Specific and nonspecific immune stimulation of MHC-II-deficient mice results in chronic HSV-1 infection of the trigeminal ganglia following ocular challenge. Virology 258:208-216. [DOI] [PubMed] [Google Scholar]
- 17.Ghiasi, H., S. L. Wechsler, S. Cai, A. B. Nesburn, and F. M. Hofman. 1998. The role of neutralizing antibody and T-helper subtypes in protection and pathogenesis of vaccinated mice following ocular HSV-1 challenge. Immunology 95:352-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghiasi, H., S. L. Wechsler, R. Kaiwar, A. B. Nesburn, and F. M. Hofman. 1995. Local expression of tumor necrosis factor alpha and interleukin-2 correlates with protection against corneal scarring after ocular challenge of vaccinated mice with herpes simplex virus type 1. J. Virol. 69:334-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guidotti, L. G., S. Guilhot, and F. V. Chisari. 1994. Interleukin-2 and alpha/beta interferon down-regulate hepatitis B virus gene expression in vivo by tumor necrosis factor-dependent and -independent pathways. J. Virol. 68:1265-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendricks, R. L., T. M. Tumpey, and A. Finnegan. 1992. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J. Immunol. 149:3023-3028. [PubMed] [Google Scholar]
- 21.Karupiah, G., A. J. Ramsay, I. A. Ramshaw, and R. V. Blanden. 1992. Recombinant vaccine vector-induced protection of athymic, nude mice from influenza A virus infection. Analysis of protective mechanisms. Scand J. Immunol. 36:99-105. [DOI] [PubMed] [Google Scholar]
- 22.Le Gros, G., S. Z. Ben-Sasson, R. Seder, F. D. Finkelman, and W. E. Paul. 1990. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J. Exp. Med. 172:921-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosmann, T. R., and R. L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 24.Nash, A. A., A. Jayasuriya, J. Phelan, S. P. Cobbold, H. Waldmann, and T. Prospero. 1987. Different roles for L3T4+ and Lyt2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J. Gen Virol. 68:825-833. [DOI] [PubMed] [Google Scholar]
- 25.Nistico, G., and G. De Sarro. 1991. Is interleukin 2 a neuromodulator in the brain? Trends Neurosci. 14:146-150. [DOI] [PubMed] [Google Scholar]
- 26.Pahwa, R., T. Chatila, S. Pahwa, C. Paradise, N. K. Day, R. Geha, S. A. Schwartz, H. Slade, N. Oyaizu, and R. A. Good. 1989. Recombinant interleukin 2 therapy in severe combined immunodeficiency disease. Proc. Natl. Acad. Sci. USA 86:5069-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul, W. E. 1989. Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell 57:521-524. [DOI] [PubMed] [Google Scholar]
- 28.Paul, W. E., and R. A. Seder. 1994. Lymphocyte responses and cytokines. Cell 76:241-251. [DOI] [PubMed] [Google Scholar]
- 29.Peace, D. J., and M. A. Cheever. 1989. Toxicity and therapeutic efficacy of high-dose interleukin 2. In vivo infusion of antibody to NK-1.1 attenuates toxicity without compromising efficacy against murine leukemia. J. Exp. Med. 169:161-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perng, G. C., E. C. Dunkel, P. A. Geary, S. M. Slanina, H. Ghiasi, R. Kaiwar, A. B. Nesburn, and S. L. Wechsler. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 68:8045-8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddehase, M. J., W. Mutter, and U. H. Koszinowski. 1987. In vivo application of recombinant interleukin 2 in the immunotherapy of established cytomegalovirus infection. J. Exp. Med. 165:650-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg, S. A., J. J. Mule, P. J. Spiess, C. M. Reichert, and S. L. Schwarz. 1985. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J. Exp. Med. 161:1169-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rouse, B. T., L. S. Miller, L. Turtinen, and R. N. Moore. 1985. Augmentation of immunity to herpes simplex virus by in vivo administration of interleukin 2. J. Immunol. 134:926-930. [PubMed] [Google Scholar]
- 34.Seder, R. A., and W. E. Paul. 1994. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 12:635-673. [DOI] [PubMed] [Google Scholar]
- 35.Siegel, J. P., and R. K. Puri. 1991. Interleukin-2 toxicity. J. Clin. Oncol. 9:694-704. [DOI] [PubMed] [Google Scholar]
- 36.Smith, K. A. 1988. Interleukin-2: inception, impact, and implications. Science 240:1169-1176. [DOI] [PubMed] [Google Scholar]
- 37.Tilden, A. B., K. Itoh, and C. M. Balch. 1987. Human lymphokine-activated killer (LAK) cells: identification of two types of effector cells. J. Immunol. 138:1068-1073. [PubMed] [Google Scholar]
- 38.Weinberg, A., and T. C. Merigan. 1988. Recombinant interleukin 2 as an adjuvant for vaccine-induced protection. Immunization of guinea pigs with herpes simplex virus subunit vaccines. J. Immunol. 140:294-299. [PubMed] [Google Scholar]
- 39.Wilhelmus, K. R., C. R. Dawson, B. A. Barron, P. Bacchetti, L. Gee, D. B. Jones, H. E. Kaufman, J. Sugar, R. A. Hyndiuk, P. R. Laibson, R. D. Stulting, and P. A. Asbell. 1996. Risk factors for herpes simplex virus epithelial keratitis recurring during treatment of stromal keratitis or iridocyclitis. Herpetic Eye Disease Study Group. Br. J. Ophthalmol. 80:969-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zwaagstra, J. C., H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1991. Identification of a major regulatory sequence in the latency associated transcript (LAT) promoter of herpes simplex virus type 1 (HSV-1). Virology 182:287-297. [DOI] [PubMed] [Google Scholar]