Abstract
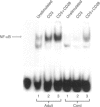
The capacity of neonatal T cells to secrete interleukin-2 (IL-2) has been reported to be variable. We analysed IL-2 production in purified neonatal and adult T cells using polyclonal activator phorbol ester + calcium ionophore (PDBu + iono) or receptor-mediated anti-CD3/anti-CD3+ anti-CD28 stimulation. PDBu + iono induced equally high IL-2 levels in both groups and, when stimulated with plate-bound anti-CD3 monoclonal antibody (mAb), the IL-2 secretion by neonatal cells was undetectable and adult cells produced low amounts of IL-2 (mean 331 +/- 86 pg/ml). The addition of anti-CD28 mAb to anti-CD3-stimulated cells markedly increased IL-2 production in both cell types, but levels of IL-2 in neonatal T cells remained clearly lower than those of adult T cells (respective mean values: 385 +/- 109 pg/ml and 4494 +/- 1199 pg/ml). As NF-kappa B is a critical transcription factor in the control of IL-2 expression, we next analysed its nuclear translocation in neonatal and adult T cells using the electrophoretic mobility shift assay and, because induction of reactive oxygen intermediates (ROI) is required for the activation of NF-kappa B, we also analysed levels of intracellular ROI in these cells using the ROI-reactive fluorochrome DCFH-DA and flow cytometry. In neonatal T cells NF-kappa B activation and ROI formation after anti-CD3 stimulation were low compared with adult T cells and, although addition of anti-CD28 mAb increased induction of NF-kappa B and ROI formation, levels similar to those of adults were not achieved. After PDBu + iono stimulation, the cells showed similar ROI formation and IL-2 secretion. Our results suggest that reduced IL-2 production by neonatal T cells is specific for anti-CD3 and anti-CD3+ anti-CD28-mediated stimulation and that these activators cannot effectively activate the ROI-NF-kappa B signalling pathway in neonatal T cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Ghanei A., Hamilton K. Up-regulation of murine neonatal T helper cell responses by accessory cell factors. J Immunol. 1994 Oct 15;153(8):3378–3385. [PubMed] [Google Scholar]
- Adkins B., Hamilton K. Freshly isolated, murine neonatal T cells produce IL-4 in response to anti-CD3 stimulation. J Immunol. 1992 Dec 1;149(11):3448–3455. [PubMed] [Google Scholar]
- Ahmed R., Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996 Apr 5;272(5258):54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Beverley P. C. Human T-cell memory. Curr Top Microbiol Immunol. 1990;159:111–122. doi: 10.1007/978-3-642-75244-5_7. [DOI] [PubMed] [Google Scholar]
- Burdon R. H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995 Apr;18(4):775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- Burow S., Valet G. Flow-cytometric characterization of stimulation, free radical formation, peroxidase activity and phagocytosis of human granulocytes with 2,7-dichlorofluorescein (DCF). Eur J Cell Biol. 1987 Feb;43(1):128–133. [PubMed] [Google Scholar]
- Chheda S., Palkowetz K. H., Garofalo R., Rassin D. K., Goldman A. S. Decreased interleukin-10 production by neonatal monocytes and T cells: relationship to decreased production and expression of tumor necrosis factor-alpha and its receptors. Pediatr Res. 1996 Sep;40(3):475–483. doi: 10.1203/00006450-199609000-00018. [DOI] [PubMed] [Google Scholar]
- Clerici M., DePalma L., Roilides E., Baker R., Shearer G. M. Analysis of T helper and antigen-presenting cell functions in cord blood and peripheral blood leukocytes from healthy children of different ages. J Clin Invest. 1993 Jun;91(6):2829–2836. doi: 10.1172/JCI116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello R., Lipcey C., Algarté M., Cerdan C., Baeuerle P. A., Olive D., Imbert J. Activation of primary human T-lymphocytes through CD2 plus CD28 adhesion molecules induces long-term nuclear expression of NF-kappa B. Cell Growth Differ. 1993 Apr;4(4):329–339. [PubMed] [Google Scholar]
- Fairfax C. A., Borzy M. S. Interleukin 2 production, proliferative response, and receptor expression by cord blood mononuclear cells. J Clin Lab Immunol. 1988 Oct;27(2):63–67. [PubMed] [Google Scholar]
- Fraser J. D., Irving B. A., Crabtree G. R., Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991 Jan 18;251(4991):313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Gerli R., Bertotto A., Crupi S., Arcangeli C., Marinelli I., Spinozzi F., Cernetti C., Angelella P., Rambotti P. Activation of cord T lymphocytes. I. Evidence for a defective T cell mitogenesis induced through the CD2 molecule. J Immunol. 1989 Apr 15;142(8):2583–2589. [PubMed] [Google Scholar]
- Ghosh P., Tan T. H., Rice N. R., Sica A., Young H. A. The interleukin 2 CD28-responsive complex contains at least three members of the NF kappa B family: c-Rel, p50, and p65. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1696–1700. doi: 10.1073/pnas.90.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan J., O'Neill S., O'Neill L. A., Pattison U., Reen D. J. Signalling via CD28 of human naive neonatal T lymphocytes. Clin Exp Immunol. 1995 Oct;102(1):192–198. doi: 10.1111/j.1365-2249.1995.tb06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan J., Reen D. J. Cord blood CD4+ CD45RA+ T cells achieve a lower magnitude of activation when compared with their adult counterparts. Immunology. 1997 Mar;90(3):397–401. doi: 10.1111/j.1365-2567.1997.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan J., Reen D. J. Reduced primary antigen-specific T-cell precursor frequencies in neonates is associated with deficient interleukin-2 production. Immunology. 1996 Apr;87(4):604–608. doi: 10.1046/j.1365-2567.1996.476587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohashi N., Hayashi T., Fusaki N., Takeuchi M., Higurashi M., Okamoto T., Semba K., Yamamoto T. The protein tyrosine kinase Fyn activates transcription from the HIV promoter via activation of NF kappa B-like DNA-binding proteins. Int Immunol. 1995 Nov;7(11):1851–1859. doi: 10.1093/intimm/7.11.1851. [DOI] [PubMed] [Google Scholar]
- Imbert V., Rupec R. A., Livolsi A., Pahl H. L., Traenckner E. B., Mueller-Dieckmann C., Farahifar D., Rossi B., Auberger P., Baeuerle P. A. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell. 1996 Sep 6;86(5):787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- Kuiper H., Brouwer M., de Boer M., Parren P., van Lier R. A. Differences in responsiveness to CD3 stimulation between naive and memory CD4+ T cells cannot be overcome by CD28 costimulation. Eur J Immunol. 1994 Sep;24(9):1956–1960. doi: 10.1002/eji.1830240903. [DOI] [PubMed] [Google Scholar]
- Lewis D. B., Yu C. C., Meyer J., English B. K., Kahn S. J., Wilson C. B. Cellular and molecular mechanisms for reduced interleukin 4 and interferon-gamma production by neonatal T cells. J Clin Invest. 1991 Jan;87(1):194–202. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los M., Dröge W., Schulze-Osthoff K. Inhibition of activation of transcription factor AP-1 by CD28 signalling in human T-cells. Biochem J. 1994 Aug 15;302(Pt 1):119–123. doi: 10.1042/bj3020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los M., Schenk H., Hexel K., Baeuerle P. A., Dröge W., Schulze-Osthoff K. IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J. 1995 Aug 1;14(15):3731–3740. doi: 10.1002/j.1460-2075.1995.tb00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo M. G., Bigler M., Ciuti E., Martino S., Tovo P. A. Immune responses by cord blood cells. Blood Cells. 1994;20(2-3):573–586. [PubMed] [Google Scholar]
- Rudd C. E., Janssen O., Cai Y. C., da Silva A. J., Raab M., Prasad K. V. Two-step TCR zeta/CD3-CD4 and CD28 signaling in T cells: SH2/SH3 domains, protein-tyrosine and lipid kinases. Immunol Today. 1994 May;15(5):225–234. doi: 10.1016/0167-5699(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Rudd C. E. Upstream-downstream: CD28 cosignaling pathways and T cell function. Immunity. 1996 Jun;4(6):527–534. doi: 10.1016/s1074-7613(00)80479-3. [DOI] [PubMed] [Google Scholar]
- Schieven G. L., Kirihara J. M., Myers D. E., Ledbetter J. A., Uckun F. M. Reactive oxygen intermediates activate NF-kappa B in a tyrosine kinase-dependent mechanism and in combination with vanadate activate the p56lck and p59fyn tyrosine kinases in human lymphocytes. Blood. 1993 Aug 15;82(4):1212–1220. [PubMed] [Google Scholar]
- Schulze-Osthoff K., Los M., Baeuerle P. A. Redox signalling by transcription factors NF-kappa B and AP-1 in lymphocytes. Biochem Pharmacol. 1995 Sep 7;50(6):735–741. doi: 10.1016/0006-2952(95)02011-z. [DOI] [PubMed] [Google Scholar]
- Sen C. K., Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996 May;10(7):709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- Shu U., Demeure C. E., Byun D. G., Podlaski F., Stern A. S., Delespesse G. Interleukin 12 exerts a differential effect on the maturation of neonatal and adult human CD45R0- CD4 T cells. J Clin Invest. 1994 Oct;94(4):1352–1358. doi: 10.1172/JCI117469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder T. F., Clement L. T., Cooper M. D. Human lymphocyte differentiation antigens HB-10 and HB-11. I. Ontogeny of antigen expression. J Immunol. 1985 May;134(5):2983–2988. [PubMed] [Google Scholar]
- Thompson C. B., Lindsten T., Ledbetter J. A., Kunkel S. L., Young H. A., Emerson S. G., Leiden J. M., June C. H. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K. The role of cytosolic Ca2+ in cell injury, necrosis and apoptosis. Curr Opin Cell Biol. 1992 Apr;4(2):227–232. doi: 10.1016/0955-0674(92)90037-d. [DOI] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Umlauf S. W., Beverly B., Kang S. M., Brorson K., Tran A. C., Schwartz R. H. Molecular regulation of the IL-2 gene: rheostatic control of the immune system. Immunol Rev. 1993 Jun;133:177–197. doi: 10.1111/j.1600-065x.1993.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Vint I. A., Foreman J. C., Chain B. M. The gold anti-rheumatic drug auranofin governs T cell activation by enhancing oxygen free radical production. Eur J Immunol. 1994 Sep;24(9):1961–1965. doi: 10.1002/eji.1830240904. [DOI] [PubMed] [Google Scholar]
- Waugh R., Clark G., Vaux P., Brown J. W. Sequence and expression of potato U2 snRNA genes. Nucleic Acids Res. 1991 Jan 25;19(2):249–256. doi: 10.1093/nar/19.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong R., Brouwer M., Miedema F., van Lier R. A. Human CD8+ T lymphocytes can be divided into CD45RA+ and CD45RO+ cells with different requirements for activation and differentiation. J Immunol. 1991 Apr 1;146(7):2088–2094. [PubMed] [Google Scholar]