Abstract
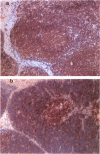
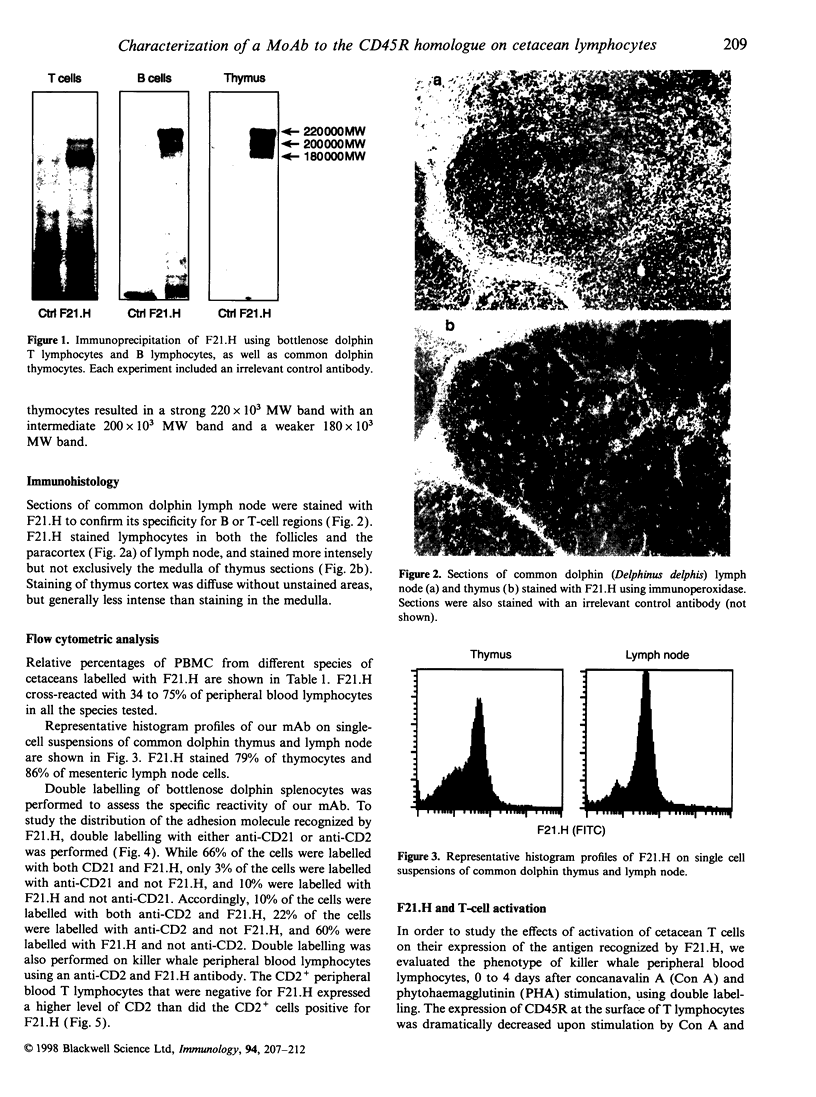
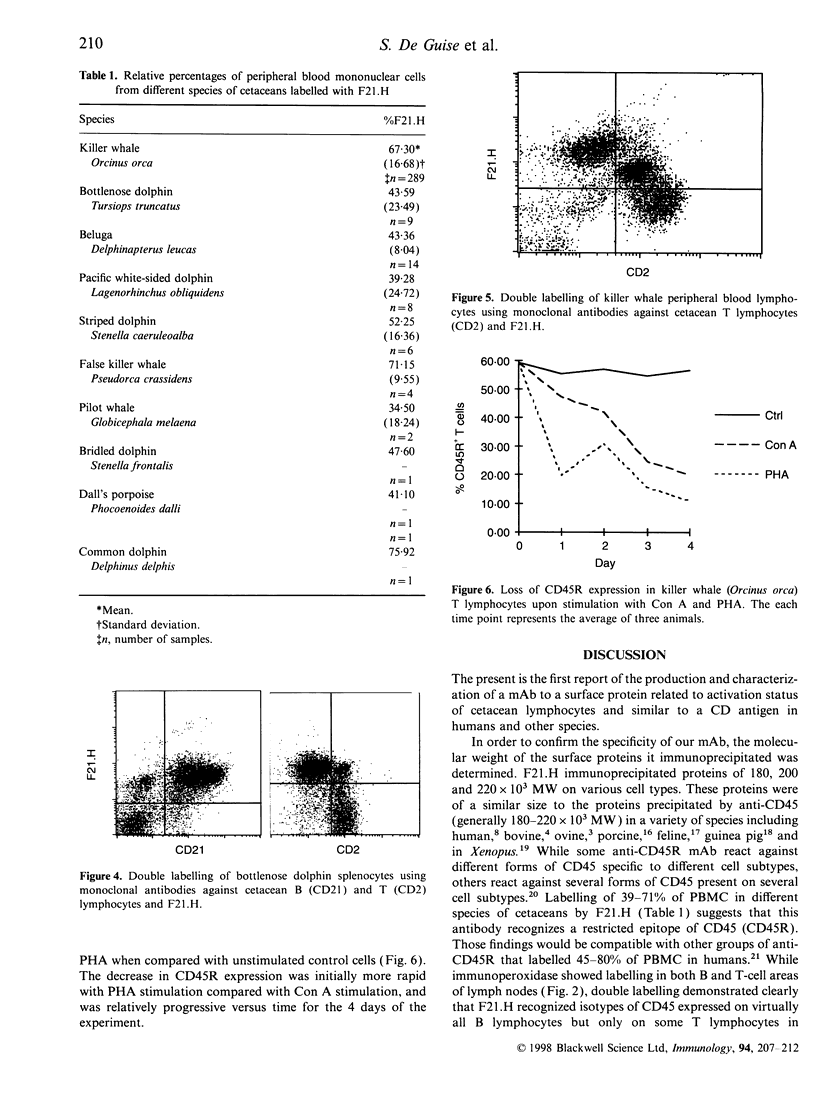
As part of our current efforts to develop assays and reagents to study the immune system of marine mammals, and in view of the effort currently made to develop monoclonal antibodies to cell surface proteins of lymphocyte subsets in different species, the present paper reports on the characterization of a monoclonal antibody against the homologue of CD45R on cetacean lymphocytes. The specificity of this antibody has been characterized on the basis of immunoprecipitation of the antigen it recognized, immunoperoxidase staining on cetacean lymph node and thymus sections, as well as one and two-colour flow cytometric analysis of cetacean peripheral blood mononuclear cells and single-cell suspensions of thymus, lymph node and spleen. Anticetacean CD45R (F21.H) immunoprecipitated proteins of 180, 200 and 220 x 10(3) MW, with the 180 x 10(3) MW from being predominantly expressed on T cells and the 220 x 10(3) MW form expressed predominantly on B cells and thymocytes F21.H labelled all B cells and a proportion of T cells on single-cell suspensions of spleen cells. CD45R- killer whale peripheral blood lymphocytes expressed a higher density of CD2 than CD45R+, a characteristic of memory T cells. Killer whale T lymphocytes also lost the expression of CD45R upon activation with concanavalin A (Con A) and phytohaemagglutinin (PHA). This is the first report of a monoclonal antibody to CD45R in cetaceans, and this antibody is foreseen as a possible valuable diagnostic and research tool to assess immune functions of captive and wild cetaceans as part of the evaluation of their health status.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Alexander D., Shiroo M., Robinson A., Biffen M., Shivnan E. The role of CD45 in T-cell activation--resolving the paradoxes? Immunol Today. 1992 Dec;13(12):477–481. doi: 10.1016/0167-5699(92)90021-X. [DOI] [PubMed] [Google Scholar]
- Barritt L. C., Turpen J. B. Characterization of lineage restricted forms of a Xenopus CD45 homologue. Dev Comp Immunol. 1995 Nov-Dec;19(6):525–536. doi: 10.1016/0145-305x(95)00031-n. [DOI] [PubMed] [Google Scholar]
- Bembridge G. P., MacHugh N. D., McKeever D., Awino E., Sopp P., Collins R. A., Gelder K. I., Howard C. J. CD45RO expression on bovine T cells: relation to biological function. Immunology. 1995 Dec;86(4):537–544. [PMC free article] [PubMed] [Google Scholar]
- Blanchard-Channell M., Moore P. F., Stott J. L. Characterization of monoclonal antibodies specific for equine homologues of CD3 and CD5. Immunology. 1994 Aug;82(4):548–554. [PMC free article] [PubMed] [Google Scholar]
- Byth K. F., Conroy L. A., Howlett S., Smith A. J., May J., Alexander D. R., Holmes N. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and B cell maturation. J Exp Med. 1996 Apr 1;183(4):1707–1718. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillitzer R., Pilarski L. M. In situ localization of CD45 isoforms in the human thymus indicates a medullary location for the thymic generative lineage. J Immunol. 1990 Jan 1;144(1):66–74. [PubMed] [Google Scholar]
- Hart I. J., Schäfer H., Scheper R. J., Stevenson G. T. Subpopulations of guinea-pig T lymphocytes defined by isoforms of the leucocyte common antigen. Immunology. 1992 Nov;77(3):377–384. [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Naessens J. Summary of workshop findings for cattle (tables 1 and 2). Vet Immunol Immunopathol. 1993 Nov;39(1-3):25–47. doi: 10.1016/0165-2427(93)90161-v. [DOI] [PubMed] [Google Scholar]
- Jacobsen C. N., Aasted B., Broe M. K., Petersen J. L. Reactivities of 20 anti-human monoclonal antibodies with leucocytes from ten different animal species. Vet Immunol Immunopathol. 1993 Dec;39(4):461–466. doi: 10.1016/0165-2427(93)90075-f. [DOI] [PubMed] [Google Scholar]
- Justement L. B., Brown V. K., Lin J. Regulation of B-cell activation by CD45: a question of mechanism. Immunol Today. 1994 Sep;15(9):399–406. doi: 10.1016/0167-5699(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law D. A., Spruyt L. L., Paterson D. J., Williams A. F. Subsets of thymopoietic rat thymocytes defined by expression of the CD2 antigen and the MRC OX-22 determinant of the leukocyte-common antigen CD45. Eur J Immunol. 1989 Dec;19(12):2289–2295. doi: 10.1002/eji.1830191217. [DOI] [PubMed] [Google Scholar]
- Li J., Campbell D., Hayward A. R. Differential response of human thymus cells to CD2 antibodies: fragmentation of DNA of CD45RO+ and proliferation of CD45RO- subsets. Immunology. 1992 Feb;75(2):305–310. [PMC free article] [PubMed] [Google Scholar]
- Lunn D. P., Holmes M. A., Antczak D. F. Summary report of the Second Equine Leucocyte Antigen Workshop. Vet Immunol Immunopathol. 1996 Nov;54(1-4):159–161. doi: 10.1016/s0165-2427(96)05674-7. [DOI] [PubMed] [Google Scholar]
- Masuoka K., Toyosaki T., Tohya Y., Norimine J., Kai C., Mikami T. Monoclonal antibodies to feline lymphocyte membranes recognize the leukocyte-common antigen (CD45R). J Vet Med Sci. 1992 Oct;54(5):865–870. doi: 10.1292/jvms.54.865. [DOI] [PubMed] [Google Scholar]
- Milinkovitch M. C., Ortí G., Meyer A. Revised phylogeny of whales suggested by mitochondrial ribosomal DNA sequences. Nature. 1993 Jan 28;361(6410):346–348. doi: 10.1038/361346a0. [DOI] [PubMed] [Google Scholar]
- Pulido R., Cebrián M., Acevedo A., de Landázuri M. O., Sánchez-Madrid F. Comparative biochemical and tissue distribution study of four distinct CD45 antigen specificities. J Immunol. 1988 Jun 1;140(11):3851–3857. [PubMed] [Google Scholar]
- Saalmüller A., Aasted B., Canals A., Dominguez J., Goldman T., Lunney J. K., Maurer S., Pescovitz M. D., Pospisil R., Salmon H. Summary of workshop findings for porcine T-lymphocyte antigens. Vet Immunol Immunopathol. 1994 Oct;43(1-3):219–228. doi: 10.1016/0165-2427(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Serra H. M., Krowka J. F., Ledbetter J. A., Pilarski L. M. Loss of CD45R (Lp220) represents a post-thymic T cell differentiation event. J Immunol. 1988 Mar 1;140(5):1435–1441. [PubMed] [Google Scholar]
- Streuli M., Hall L. R., Saga Y., Schlossman S. F., Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. C., Dellinger J. D., Cullor J. S., Stott J. L. Bovine milk lymphocytes display the phenotype of memory T cells and are predominantly CD8+. Cell Immunol. 1994 Jun;156(1):245–253. doi: 10.1006/cimm.1994.1169. [DOI] [PubMed] [Google Scholar]
- Thomas M. L. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H., Diltz C. D., Fischer E. H., Walsh K. A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988 Nov 29;27(24):8695–8701. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- Zuckermann F. A., Binns R. M., Husmann R., Yang H., Carr M. M., Kim Y. B., Davis W. C., Misfeldt M., Lunney J. K. Analyses of monoclonal antibodies reactive with porcine CD44 and CD45. Vet Immunol Immunopathol. 1994 Oct;43(1-3):293–305. doi: 10.1016/0165-2427(94)90151-1. [DOI] [PubMed] [Google Scholar]
- van Noesel C. J., Gruters R. A., Terpstra F. G., Schellekens P. T., van Lier R. A., Miedema F. Functional and phenotypic evidence for a selective loss of memory T cells in asymptomatic human immunodeficiency virus-infected men. J Clin Invest. 1990 Jul;86(1):293–299. doi: 10.1172/JCI114698. [DOI] [PMC free article] [PubMed] [Google Scholar]