Abstract
Epstein-Barr virus (EBV) latent membrane protein 2A (LMP2A) is widely expressed in both EBV-infected cells and EBV-associated malignancies. However, the function of LMP2A is still veiled. In this study, LMP2A was found to induce the kinase activities of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase/stress-activated protein kinase JNK/SAPK. Furthermore, the downstream effector c-Jun showed hyperphosphorylation under LMP2A expression. The phosphorylation could be inhibited by the ERK pathway inhibitor PD98059, indicating that ERK may contribute to the phosphorylation of c-Jun in LMP2A-expressing cells. The impact on c-Jun phosphorylation by mitogen-activated protein kinase (MAPK) is suggested to increase c-Jun protein stability, and this was also observed in LMP2A-expressing cells by a protein synthesis inhibition assay. Moreover, LMP2A-induced cell invasion was inhibited in the presence of the ERK pathway inhibitor. Taken together, we suggest that LMP2A may exploit MAPK kinases and affect both the phosphorylation and stability of c-Jun protein. Additionally, LMP2A may thereby promote the mobility of the cells. In doing so, it may enhance the mobility of EBV-infected cells and contribute to the metastatic process of malignant cells. Here we demonstrated the first evidence of LMP2A-induced migration and the underlying pathways accounting for it.
Epstein-Barr virus (EBV) belongs to the Gammaherpesviridae family and establishes lifelong persistence in infected individuals (45). EBV is able to infect B cells and epithelial cells (45). Several malignancies derived from lymphoid and epithelial origin are strongly associated with EBV, including African Burkitt's lymphoma, Hodgkin's lymphoma, and nasopharyngeal carcinoma (NPC) (45). The underlying mechanisms of how EBV persists in humans and how the virus contributes to the tumors are still veiled. But the oncogenic potential of EBV has been demonstrated in vitro by immortalizing primary B cells and epithelial cells (38, 45). The EBV-immortalized B lymphocytes are termed lymphoblastoid cell lines, and they express a restricted pattern of viral gene products, including EBNAs, latent membrane protein 1 (LMP1), LMP2, and EBV-encoded RNAs (45). In vivo, EBV is primarily latent in memory B cells and only expresses the viral LMP2 gene (45). Given that LMP2A is expressed both in vitro and in vivo, the study of its function is of great interest. In addition to the B cells, the ubiquitous expression of LMP2A transcripts in NPC biopsies and the elevation of LMP2A antibody titers in NPC sera hint at its importance in epithelial carcinomas as well (2, 4, 26).
LMP2A localizes at the plasma membrane and in the cytoplasm in EBV-infected cells (29). Sequence analysis of LMP2A reveals eight tyrosine residues within its N-terminal region, and LMP2A is shown to be highly tyrosine phosphorylated in B cells (29). These phosphotyrosines are possible sites for the binding of cellular signaling molecules that contain Src homology 2 domains. In addition to phosphotyrosine residues, LMP2A harbors multiple proline-rich regions and thus may confer binding on proteins with Src homology 3 or WW domains (29).
The breakthrough in understanding the LMP2A signaling modulation capacity is initiated in the B-cell system. In B lymphocytes, antigen cross-linking leads to the activation of the B-cell receptor (BCR) (12). The immunoglobulin α/β immunoreceptor tyrosine-based activation motif (ITAM) of the BCR is phosphorylated and in turn acts as a docking site for Syk kinase (12), and the sequences adjacent to the ITAM in the BCR can interact with Src family kinases such as Lyn (12). The kinases clustered by the BCR are then activated and stimulate downstream events such as recruitment and activation of other kinases and phosphatases, hydrolysis of phospholipids, activation of protein kinase C, and mobilization of intracellular calcium, and they eventually affect the nuclear transcription factors (12). These signals finally lead B cells to differentiation and proliferation (12).
However, BCR-mediated signal transduction is prohibited in the presence of LMP2A (33). In the N-terminal domain of LMP2A, sequences around tyrosine 74 and tyrosine 85 form a functional ITAM. This motif and the adjacent region in LMP2A are documented as required not only for association with the Lyn, Fyn, Syk, and Csk kinases but also for the inhibition of BCR signals (3, 15, 16, 29, 30, 46). LMP2A also has proline-rich motifs, which can bind to WW domain-containing Nedd-4 family ubiquitin-protein ligases (22). LMP2A is suggested to be a molecular scaffold for the recruitment of both BCR-associated tyrosine kinases and E3 protein-ubiquitin ligases, and this may prevent B cells from being activated (55). One of the consequences of BCR activation is the induction of the EBV lytic cycle, which expresses 80 to 100 EBV genes (31, 45). Many of them are highly immunogenic and may thus jeopardize the virus-infected cells at the risk of being eradicated (45). Through inhibiting BCR activation, LMP2A may have the role of sustaining the EBV in the latent stage and thereby keeping the infected cells from being recognized by the immune system (32). On the other hand, in the in vivo transgenic mouse model, the expression of LMP2A confers certain unidentified signals to help the survival and proliferation of the cells (5, 29).
In epithelial cells, by contrast, the identified signals triggered by LMP2A are more obscure than in B cells. The phosphorylation of LMP2A is dependent on the stimulation of the extracellular matrix, which suggests that LMP2A can probably initiate signaling after having attached to the extracellular matrix (46). Another report showed the transformation ability of LMP2A demonstrated by the hyperproliferative rate and anchorage independence in LMP2A-expressing HaCaT cells (47).
In light of the ample motifs harbored by LMP2A, it becomes interesting to ask whether LMP2A can also modulate signal transduction pathways and how the signal changes account for its function. Previously, LMP2A had been shown to augment the AP-1 and NF-κB pathway triggered by another EBV oncoprotein, LMP1 (10, 24, 35), but no evidence regarding the signaling provoked by LMP2A per se had ever been discovered.
Mitogen-activated protein kinases (MAPKs) not only connect the cell surface signals to the decisive nuclear factors but also act as convergence points in the signaling networks (53). The MAPK members, including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38, have great influence on the control of metabolic processes, such as proliferation, migration, differentiation, and apoptosis, under normal physiological conditions (19, 23). In view of the principal roles played by MAPK, and since LMP2A is suggested to be a signaling modulator, we then investigated the activation status of MAPK and its downstream effectors in LMP2A-expressing cells.
Characterization of LMP2A-expressing epithelial cells.
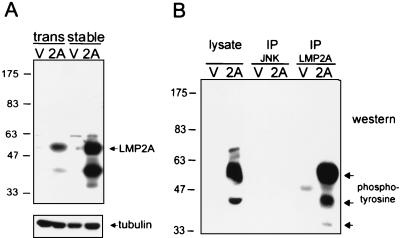
To investigate signaling changes induced by LMP2A expression, constitutive LMP2A-expressing cell line 293-LMP2A was established (18). pRC-LMP2A contains the full-length LMP2A sequence originated from pLMP2A (30). Cells were transfected by using a modified calcium phosphate method (8) and then selected with neomycin (800 μg/ml). To check the expression of LMP2A in stable clones and transient transfectants, cells were transfected with Rc/CMV LMP2A or vector plasmid pRc/CMV (Invitrogen, Groningen, The Netherlands) and lysed in 1% NP-40 buffer (50 mM Tris [pH 8.0], 137 mM NaCl, 10% glycerol, 2 mM EDTA, 50 mM sodium orthovanadate, 1% NP-40, 50 mM NaF, 0.001% leupeptin, 0.001% aprotinin, and 1 mM phenylmethylsulfonyl fluoride). Then the protein was resolved by sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis and blotted onto a polyvinylidene difluoride nitrocellulose membrane (Millipore, Bedford, Mass.). The LMP2A antibody was generated by immunizing the rabbit with full-length LMP2A (data not shown). Thus, the expression of LMP2A was confirmed by Western blot analysis with anti-LMP2A antibody (Fig. 1A). The detection of tubulin by anti-tubulin antibody (Amersham Pharmacia, Piscataway, N.J.) served as an internal control for the protein amounts. Since tyrosine phosphorylation plays a pivotal role in LMP2A function, anti-phosphotyrosine antibody (4G10; Upstate Biotechnology, Lake Placid, N.Y.) was used to evaluate the phosphorylation status of LMP2A in these cells. The major difference between LMP2A-expressing cells and the control lines was a heavily tyrosine-phosphorylated protein at around 54 kDa, the predicted mass of LMP2A (Fig. 1B). However, the possibility cannot be excluded that this signal comes from a cellular protein induced by LMP2A with the same molecular mass. Therefore, LMP2A was immunoprecipitated from the lysates by anti-LMP2A antibody and then probed with the anti-phosphotyrosine antibody in the following Western blot analysis. The same pattern was obtained (Fig. 1B). Therefore, LMP2A is suggested to be tyrosine phosphorylated in epithelial cells. According to previous reports, tyrosine phosphorylation is important for LMP2A to function in B cells (reviewed in reference 29). The presence of similar tyrosine phosphorylation indicates that LMP2A may influence some signaling in epithelial cells. Besides, an immunofluorescence assay was carried out to determine the percentage of cells expressing the LMP2A protein. More than 90% of the cells expressed LMP2A protein at the plasma membrane and in the cytoplasm (data not shown).
FIG. 1.
Characterization of LMP2A-expressing epithelial cells. (A) Demonstration of the expression of LMP2A in transient and stable transfectants by Western blot analysis. Total proteins from transient transfectants (trans) or stable clones (stable) of LMP2A (2A) and vector control (V) cells were blotted onto the polyvinylidene difluoride membrane and reacted with LMP2A-specific antibodies. The detection of tubulin served as an internal control of protein amounts. (B) Examination of the phosphorylation status of LMP2A protein. Cell lysates of LMP2A and stable vector control clones underwent immunoprecipitation (IP) with anti-LMP2A antibodies or an irrelevant antibody control (JNK). Then the immunocomplex products were denatured, subjected to electrophoresis, and blotted onto the membrane. The blots were then reacted with anti-phosphotyrosine antibodies (4G10) for Western blot analysis. The numbers on the left are markers presenting molecular mass in kilodaltons.
Investigation of endogenous MAPK activities in LMP2A-expressing cells.
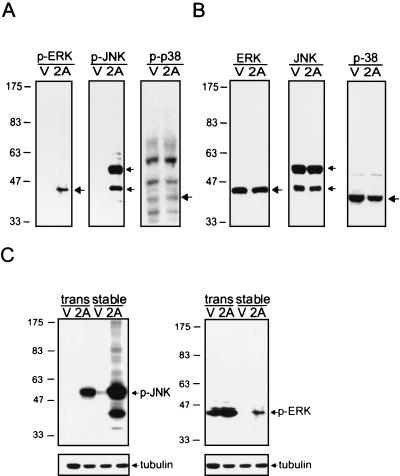
MAPK cascades are involved in a wide spectrum of key events, including proliferation, migration, differentiation, and apoptosis (7). Since LMP2A structurally resembles a signaling modulator, the possibility that LMP2A regulates the MAPK cascades was tested. Three major groups of MAPK have been identified. They are ERK, JNK, and p38 MAPK. All of them are activated by being phosphorylated at specific amino acids. Activation of ERK occurs through phosphorylation of Thr 202 and Tyr 204 (40). JNK is activated by phosphorylation on Thr 183 and Tyr 185 (13). p38 is activated when Thr 180 and Tyr 182 have been phosphorylated (44). Therefore, phosphoprotein-specific antibodies (Cell Signaling Technology, Beverly, Mass.) were used to evaluate the activation status of these endogenous MAPKs. Some differential activation patterns were observed between LMP2A and vector control cells: phospho-ERK was present in LMP2A-expressing lines but not in vector control lines (Fig. 2A). The same pattern was also detected in another epithelial cell line, NPC-TW01 (data not shown). In addition, LMP2A prominently increased the amounts of phospho-JNK (Fig. 2A), but no obvious activation of p38 MAPK was observed (Fig. 2A). Besides, no changes occurred when detecting the total amount of JNK and ERK (Fig. 2B). The two phosphorylated products of JNK are of different isoforms. Until now, no functional difference between these isoforms had been discovered (52). Besides, in order to rule out the possibility of clonal variation, calcium-based transient transfection was also carried out. Consistently, phospho-ERK and phospho-JNK were increased in LMP2A transfectants only but not in vector control transfectants (Fig. 2C). Therefore, LMP2A could regulate the activation of ERK and JNK but not the amount of each.
FIG. 2.
Detection of the phosphorylation status of three major components of MAPK families (ERK, JNK, and p38) in LMP2A-expressing cells. (A) Western blot analyses with phosphospecific ERK (p-ERK), JNK (p-JNK), and p38 (p-p38) antibodies were performed to detect the phosphorylation status of these MAPKs in stable LMP2A (2A) and vector control (V) clones. (B) Total amounts of ERK, JNK, and p38 were also detected. (C) Total proteins from transient transfectants (trans) or stable clones (stable) of LMP2A (2A) and vector control (V) were blotted on the membrane and reacted with anti-phosphorylated JNK (p-JNK) antibody and anti-phosphorylated ERK (p-ERK) antibody. The detection of tubulin served as an internal control of protein amounts. The numbers to the left of each panel represent molecular mass in kilodaltons.
Phosphorylation status of MAPK downstream effectors.
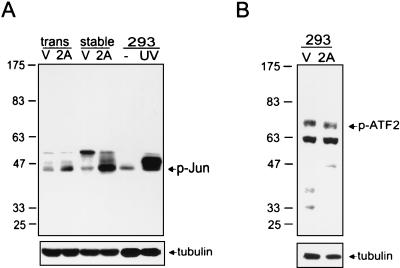
Since ERK and JNK are activated in response to LMP2A expression, we next examined the downstream effectors of ERK and JNK (13, 27, 34, 41). The levels of phospho-c-Jun (Ser 63), and phospho-ATF2 (activating transcription factor 2) were detected with anti-phospho-c-Jun (Santa Cruz, Santa Cruz, Calif.) and anti-phospho-ATF2 antibodies (Cell Signaling Technology). Apparently, phospho-c-Jun was increased in LMP2A stable clones and transient transfectants (Fig. 3A), whereas the level of ATF2 was constant (Fig. 3B). The phosphorylation of c-Jun is also elevated in LMP2A-expressing NPC-TW01 cells (data not shown).
FIG. 3.
Investigation of the phosphorylation status of MAPK downstream effectors in LMP2A-expressing clones. (A) Antibodies that specifically react with phospho-c-Jun were used to investigate the phosphorylation of c-Jun in stably expressing clones (stable) and transient transfectants (trans) in LMP2A-expressing (2A) and vector control (V) cells. UV-treated (200 J/m2) 293 cells (UV) served as the positive controls for phospho-c-Jun induction. (B) Antibodies that specifically react with phospho-ATF2 were used to investigate the phosphorylation of ATF2 in LMP2A-expressing (2A) and vector control (V) cells. The detection of tubulin served as an internal control of protein amounts. The numbers to the left of each panel represent molecular mass in kilodaltons.
The involvement of ERK in LMP2A-mediated c-Jun phosphorylation.
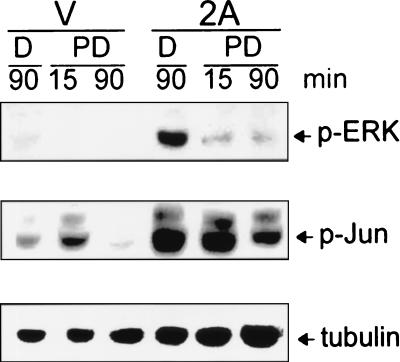
To clarify whether LMP2A increases c-Jun phosphorylation through the activation of ERK, the ERK pathway inhibitor PD98059 (Calbiochem, San Diego, Calif.) was used (11). Cells were seeded on six-well plates (4 × 105 cells/well) and starved for 12 h. The cells were then treated with 100 μM PD98059 for 30 min. Apparently, PD98059 inhibited LMP2A-induced phosphorylation of c-Jun coinciding with inhibition of ERK phosphorylation (Fig. 4). In the presence of PD98059, the phosphorylated JNK remained in LMP2A-expressing cells, showing the specificity of the inhibitor (data not shown). The dimethyl sulfoxide (DMSO) and tubulin controls excluded the possibility of signals induced by the DMSO dissolvent or unequal amounts of protein loading. Therefore, the phosphorylation of c-Jun is in part contributed by ERK.
FIG. 4.
Involvement of the ERK pathway in LMP2A-induced c-Jun phosphorylation. Both the 293 LMP2A-expressing (2A) and vector control (V) stable clones were treated with DMSO (D) or ERK pathway inhibitor PD98059 (PD) for 30 min. After 15 or 90 min of incubation in the presence of 10% fetal bovine serum, the cells were harvested. Western blot analyses were performed with anti-phospho-ERK (p-ERK) antibody, anti-phospho-Jun (p-Jun) antibody, and anti-tubulin antibody.
Detection of c-Jun stability in LMP2A-expressing cells.
The action of phosphorylation on c-Jun not only increases its DNA binding ability but also prevents c-Jun from degradation (17, 36). In LMP2A-expressing cells, ERK/JNK and its downstream substrate c-Jun were both phosphorylated. We then tested whether c-Jun gains more stability under LMP2A expression. Actually, the expression level of c-Jun was higher in LMP2A-expressing cells; this also hints at the possibility of an increase in stability (Fig. 5A). Cycloheximide (35 μM; Sigma, St. Louis, Mo.) was added to the vector and LMP2A-expressing cells to block de novo protein synthesis. Then the stability of preformed c-Jun was measured at the indicated periods. The amounts of c-Jun decreased dramatically in the control cells while the amounts of c-Jun in LMP2A-expressing cells persisted (Fig. 5B). Therefore, the expression of LMP2A can also modulate the stability of c-Jun.
FIG. 5.
Examination of the stability of c-Jun protein in LMP2A and vector control cells. (A) Total amounts of c-Jun protein in LMP2A-expressing (2A) and vector control (V) cells were detected with anti-c-Jun antibodies by Western blot analysis. (B) Both LMP2A-expressing (2A) and vector control (V) cells were treated with 10 μg of cycloheximide/ml to inhibit protein synthesis. Cell lysates were harvested at each indicated time and subjected to Western blot analysis with anti-Jun antibodies. The detection of tubulin served as an internal control of protein amounts. The numbers to the left of each panel represent molecular mass in kilodaltons.
Involvement of the ERK pathway in LMP2A-mediated cell mobility.
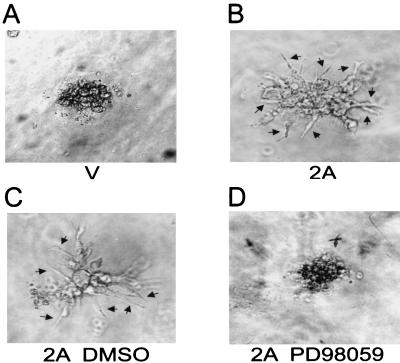
The ERK pathway is important for the migration of breast cancer cells, fibrosarcoma cells, and endothelial cells (9, 28, 37). Therefore, a three-dimensional collagen gel assay was carried out to investigate the possible effects elicited by LMP2A. In brief, cells (8 × 103 cells) were resuspended in a collagen gel mixture (70 μl of 3-mg/ml rat tail collagen I [Sigma], 9 μl of 10× Dulbecco's minimal essential medium, 2 μl of 0.2 M HEPES [pH 7.3], and 5 μl of H2O, with the pH adjusted to around 7.4 with 5 N NaOH). The cells were moved into a 37°C incubator with 5% CO2 for 30 min. Then, 100 μl of 1× Dulbecco's minimal essential medium containing 2.5% fetal calf serum was added. After 14 days of incubation, the phenotypes were observed and photographed by an inverted photomicroscope (Axiovert 10; Zeiss, Oberkochen, Germany). LMP2A-expressing cells extended into the collagen gel and exhibited a spindle-like shape in a three-dimensional environment while vector control cells exhibited a spherical shape in the collagen gel (Fig. 6A and B). This invasiveness phenotype could be blocked by the ERK pathway inhibitor PD98059 but not by the DMSO control (Fig. 6C and D). Therefore, activated ERK may account for LMP2A-mediated cell migration.
FIG. 6.
Demonstration of the invasion ability of LMP2A-expressing cells in a three-dimensional collagen gel. Cells transfected with vector control (A) and LMP2A expression plasmid (B) were embedded in a type I collagen gel. After 14 days of incubation, the invasion phenotypes were observed and are highlighted with arrows. To explore the involvement of the ERK pathway in this invasion behavior, DMSO control (C) and ERK inhibitor PD98059-treated (D) LMP2A-expressing cells were also embedded in the collagen gel for comparison.
LMP2A is often detected in EBV-associated epithelial pathologies, such as NPC and oral hairy leukoplakia (2, 4, 54). But the consequences of this viral gene expression in epithelial cells, especially the changes it brings in intracellular signaling proteins, are still waiting to be uncovered.
By immunoprecipitation and Western analysis, it was demonstrated that LMP2A is heavily tyrosine phosphorylated (Fig. 1). LMP2A-expressing cells were shown to have elevated JNK and ERK activities in comparison to the vector control cells (Fig. 2). Their common downstream effector c-Jun was also phosphorylated (Fig. 3A). Moreover, this activation of ERK and c-Jun decreased in the presence of the ERK pathway inhibitor PD98059 (Fig. 4), indicating that the phosphorylation of Jun was partially dependent on ERK activation. However, the incomplete PD98059 inhibition of c-Jun phosphorylation raises the possibility of JNK involvement.
At the posttranslational level, c-Jun activity could be regulated by activated ERK and JNK (43). Phosphorylation on Ser 63 has been suggested to reduce the ubiquitination of c-Jun protein and thereby retard its degradation (17, 36). The phosphorylation on c-Jun was increased in LMP2A-expressing cells and makes us wonder how stable c-Jun is (Fig. 3A). In the presence of a protein synthesis inhibitor, the c-Jun protein, which is influenced by LMP2A, was strikingly steadier than in the control cells (Fig. 5B). The data probably had delineated an LMP2A pathway beginning with the activation of the MAPK kinases and changing the phosphorylation state of c-Jun, and it may thereby stabilize it from rapid turnover. Most interestingly, LMP2A does exhibit features distinct from those previously documented in B cells. It was suggested that LMP2A might enhance the degradation of BCR-associated kinases through the interaction between LMP2A PY motifs and Nedd4 family ubiquitin-protein ligases (21, 22, 55). Taken together, the data indicate that LMP2A may exert distinct effects on protein stability through different mechanisms.
What is the consequence of c-Jun phosphorylation regulated by LMP2A? The phosphorylated amino acids 63 and 73 are located in the δ domain of c-Jun (51). The δ domain is the region that intercedes in unphosphorylated c-Jun multiubiquitination and degradation (51). Interestingly, the aggressive viral oncoprotein v-Jun is deleted within these sequences and becomes more stable than c-Jun, indicating that the stability of Jun may contribute to its oncogenicity (20, 51). The phosphorylation and stability of c-Jun are enhanced by LMP2A, which suggests that some Jun-mediated signaling probably occurs in these cells. Under mitogen stimulation, c-Jun is induced as an immediate early factor, suggesting a role in the promotion of cell growth (1, 25). Furthermore, Jun harbors oncogenicity in that it is capable of transforming chicken embryo fibroblasts (6, 56). Additionally, in mammalian cell cultures, c-Jun can cooperate with another factor, such as activated Ras, to carry out effective transformation (48, 49). c-Jun is also a strong inhibitor of differentiation (42, 50). LMP2A has been shown to transform and inhibit the differentiation of keratinocytes in organic raft cultures (47) and also to promote proliferation in B cells; therefore, it would be worthwhile to check whether the potency comes from c-Jun activation in those systems.
In addition to its mitogenic role, the ERK pathway is also important in cell migration, as demonstrated in migration and invasion in breast cancer, fibrosarcoma, and endothelial cells (9, 28, 37). ERK activation could be triggered by integrin engagement, growth factors, and v-Src expression (14, 37, 57). In our study, we also found that LMP2A could induce cell invasion through the ERK pathway (Fig. 6). In light of the high metastasis rate of NPC and the high expression of LMP2A in it, LMP2A may contribute to the metastatic property of these malignant cells.
How does LMP2A regulate the activation of MAPK? In the B-cell system, LMP2A is described as a molecular scaffold for the recruitment of tyrosine kinases (55). The abundance of functional motifs within the LMP2A N terminus contributes to this potency. The identification of an LMP2A-interacting protein or the use of serial mutant constructs in the putative interacting motifs may give clues as to which effectors are involved in this LMP2A/MAPK/c-Jun pathway. Furthermore, LMP2A has been shown to be phosphorylated by ERK (39), and there may exist a reaction circuit between LMP2A and ERK. In our lab, we previously used a microarray assay to examine the genes transcriptionally regulated by LMP2A (data not shown). A putative G protein-coupled receptor (GPCR) is upregulated under LMP2A expression (data not shown). Since the GPCR could be one of the upstream effectors of the MAPK (19), the possibility of a closer linkage between this GPCR to MAPK/c-Jun is currently being researched.
Acknowledgments
We are indebted to Richard Longnecker (Northwestern University Medical School, Chicago, Ill.) for providing the LMP2A-expressing plasmids and for valuable discussions. We thank Chun-Chih Tan for technical assistance.
This work was supported by the National Science Council (grants NSC89-2320-B-002-057, NSC 90-2318-B-002-005-M51, and NSC 91-3112-B-002-016).
REFERENCES
- 1.Angel, P., and M. Karin. 1991. The role of Jun, Fos, and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1072:129-157. [DOI] [PubMed] [Google Scholar]
- 2.Brooks, L., Q. Y. Yao, A. B. Rickinson, and L. S. Young. 1992. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J. Virol. 66:2689-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkhardt, A. L., J. B. Bolen, E. Kieff, and R. Longnecker. 1992. An Epstein-Barr virus transformation-associated membrane protein interacts with src family tyrosine kinase. J. Virol. 66:5161-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busson, P., R. McCoy, R. Sadler, K. Grilligan, T. Tursz, and N. Rabb-Traub. 1992. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J. Virol. 66:3257-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell, R. G., J. B. Wilson, S. J. Anderson, and R. Longnecker. 1998. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 9:405-411. [DOI] [PubMed] [Google Scholar]
- 6.Castellazzi, M., J. P. Dangy, F. Mechta, S. Hirai, M. Yaniv, J. Samarut, A. Lassailly, and G. Brun. 1990. Overexpression of avian or mouse c-jun in primary chick embryo fibroblasts confers a partially transformed phenotype. Oncogene 5:1541-1547. [PubMed] [Google Scholar]
- 7.Chang, L., and M. Karin. 2001. Mammalian MAPK kinase signaling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 8.Chen, C., and H. Okayama. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7:2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho, S. Y., and R. L. Klemke. 2000. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J. Cell Biol. 149:223-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson, C. W., J. H. George, S. M. S. Blake, R. Longnecker, and L. S. Young. 2001. The Epstein-Barr virus encoded latent membrane protein 2A augments signaling from latent membrane protein 1. Virology 289:192-207. [DOI] [PubMed] [Google Scholar]
- 11.Deak, M., A. D. Clifton, J. M. Lucocq, and D. R. Alessi. 1998. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17:4426-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFranco, A. L. 1997. The complexity of signaling pathways activated by the BCR. Curr. Opin. Immunol. 9:296-308. [DOI] [PubMed] [Google Scholar]
- 13.Derijard, B., M. Hibi, I. H. Wu, T. Barrett, B. Su, T. Deng, M. Karin, and R. J. Davis. 1994. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025-1037. [DOI] [PubMed] [Google Scholar]
- 14.Fincham, V. J., M. James, M. C. Frame, and S. J. Winder. 2000. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 19:2911-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fruehling, S., and R. Longnecker. 1997. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology 235:241-251. [DOI] [PubMed] [Google Scholar]
- 16.Fruehling, S., R. Swart, K. M. Dolwick, E. Kremmer, and R. Longnecker. 1998. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J. Virol. 72:7796-7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs, S. Y., L. Dolan, R. J. Davis, and Z. Ronai. 1996. Phosphorylation dependent targeting of c-Jun ubiquitination by Jun N-kinase. Oncogene 13:1531-1535. [PubMed] [Google Scholar]
- 18.Graham, F. L., and J. Smiley. 1977. Characterization of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-72. [DOI] [PubMed] [Google Scholar]
- 19.Gutkind, J. S. 1998. Cell growth control by G protein-coupled receptors: from signal transduction to signal integration. Oncogene 17:1331-1342. [DOI] [PubMed] [Google Scholar]
- 20.Hibi, M., A. Lin, T. Smeal, A. Minden, and M. Karin. 1993. Identification of an oncoprotein and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 7:2135-2148. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda, M., A. Ikeda, and R. Longnecker. 2000. PY motifs of Epstein-Barr virus LMP2A regulate protein stability and phosphorylation of LMP2A-associated proteins. J. Virol. 75:5711-5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeda, M., A. Ikeda, L. C. Longan, and R. Longnecker. 2000. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology 268:178-191. [DOI] [PubMed] [Google Scholar]
- 23.Ip, Y. T., and R. J. Davis. 1998. Signal transduction by the c-Jun N-terminal kinase (JNK)-from inflammation to development. Curr. Opin. Cell Biol. 10:205-219. [DOI] [PubMed] [Google Scholar]
- 24.Kieser, A., E. Kilger, O. Gires, M. Ueffing, W. Kolch, and W. Hammerschmidt. 1997. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 16:6478-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovary, K., and R. Bravo. 1991. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol. Cell. Biol. 11:4466-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lennette, E. T., G. Winberg, M. Yadav, G. Enblad, and G. Klein. 1995. Antibodies to LMP2A/2B in EBV-carrying malignancies. Eur. J. Cancer 31A:1875-1878. [DOI] [PubMed] [Google Scholar]
- 27.Leppä, S., R. Saffrich, W. Ansorge, and D. Bohmann. 1998. Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. EMBO J. 17:4404-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lian, J., C. Marcinkiewicz, S. Niewiarowski, and D. A. Beacham. 2001. Extracellular signal-regulated kinase (ERK) activation is required for GP Ibalpha-dependent endothelial cell migration. Thromb. Haemost. 86:1555-1562. [PubMed] [Google Scholar]
- 29.Longnecker, R. 2000. Epstein-Barr virus latency: LMP2, a regulator or means for Epstein-Barr virus persistence? Adv. Cancer Res. 79:175-200. [DOI] [PubMed] [Google Scholar]
- 30.Longnecker, R., B. Druker, T. M. Roberts, and E. Kieff. 1991. An Epstein-Barr virus membrane protein associated with cell growth transformation interacts with a tyrosine kinase. J. Virol. 65:3681-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, C. L., A. L. Burkhardt, J. H. Lee, B. Stealey, R. Longnecker, J. B. Bolen, and E. Kieff. 1995. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity 2:155-166. [DOI] [PubMed] [Google Scholar]
- 32.Miller, C. L., J. H. Lee, E. Kieff, and R. Longnecker. 1994. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc. Natl. Acad. Sci. USA 91:772-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, C. L., R. Longnecker, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J. Virol. 67:3087-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minden, A., A. Lin, T. Smeal, B. Derijard, M. Cobb, R. Davis, and M. Karin. 1994. c-Jun N-terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen-activated protein kinases. Mol. Cell. Biol. 14:6683-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murono, S., T. Yoshizaki, H. Sato, H. Takeshita, M. Furukawa, and J. S. Pagano. 2000. Aspirin inhibits tumor cell invasiveness induced by Epstein-Barr virus latent membrane protein 1 through suppression of matrix metalloproteinase-9 expression. Cancer Res. 60:2555-2561. [PubMed] [Google Scholar]
- 36.Musti, A. M., M. Treier, and D. Bohmann. 1997. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAPK kinases. Science 275:400-402. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen, D. H., A. D. Catling, D. J. Webb, M. Sankovic, L. A. Walker, A. V. Somlyo, M. J. Weber, and S. L. Gonias. 1999. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J. Cell Biol. 146:149-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishikawa, J., S. Imai, T. Oda, T. Kojima, K. Okita, and K. Takada. 1999. Epstein-Barr virus promotes epithelial cell growth in the absence of EBNA2 and LMP1 expression. J. Virol. 73:1286-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panousis, C. G., and D. T. Rowe. 1997. Epstein-Barr virus latent membrane protein 2A associates with and is a substrate for mitogen-activated protein kinase. J. Virol. 71:4752-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Payne, D. M., A. J. Rossomando, P. Martino, A. K. Erickson, J. H. Her, J. Shabanowitz, D. F. Hunt, M. J. Weber, and T. W. Sturgill. 1991. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 10:885-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peverali, F. A., A. Isaksson, A. A. Papavassiliou, P. Plastina, L. M. Staszewski, M. Mlodzik, and D. Bohmann. 1996. Phosphorylation of Drosophila Jun by the MAP kinase rolled regulates photoreceptor differentiation. EMBO J. 15:3943-3950. [PMC free article] [PubMed] [Google Scholar]
- 42.Prochownik, E. V., M. J. Smith, K. Snyder, and D. Emeagwali. 1990. Amplified expression of three jun family members inhibits erythroleukemia differentiation. Blood 76:1830-1837. [PubMed] [Google Scholar]
- 43.Rahmsdorf, H. J. 1996. Jun: transcription factor and oncoprotein. J. Allergy Clin. Immunol. 98:S183-S191.8977526 [Google Scholar]
- 44.Raingeaud, J., A. J. Whitmarsh, T. Barrett, B. Derijard, and R. J. Davis. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus and its replication, p. 2511-2573. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamp, M. A. Martin, B. Roizmam, and S. E. Straus (ed.), Virology, 4th ed. Lippincott, Williams, and Wilkins, Philadelphia, Pa.
- 46.Scholle, F. S., R. Longnecker, and N. Raab-Traub. 1999. Epithelial cell adhesion to extracellular matrix proteins induces tyrosine phosphorylation of the Epstein-Barr virus latent membrane protein 2: a role for C-terminal Src kinase. J. Virol. 73:4767-4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scholle, F. S., M. Katharine, K. M. Bendt, and N. Raab-Traub. 2000. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 74:10681-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schutte, J., J. D. Minna, and M. J. Birrer. 1989. Deregulated expression of human c-jun transforms primary rat embryo cells in cooperation with an activated c-Ha-ras gene and transforms rat-1a cells as a single gene. Proc. Natl. Acad. Sci. USA 86:2257-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smeal, T., B. Binetruy, D. A. Mercola, and M. Karin. 1991. Oncogenic and transcriptional cooperation with Ha-ras requires phosphorylation of c-jun on serine 63 and 73. Nature 354:494-496. [DOI] [PubMed] [Google Scholar]
- 50.Su, H. Y., T. J. Bos, F. S. Monteclaro, and P. K. Vogt. 1991. Jun inhibits myogenic differentiation. Oncogene 6:1759-1766. [PubMed] [Google Scholar]
- 51.Treier, M., L. M. Staszewski, and D. Bohmann. 1994. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell 78:787-798. [DOI] [PubMed] [Google Scholar]
- 52.Wang, T. H., H. S. Wang, and Y. K. Soong. 2000. Regulation and functions of c-Jun N-terminal kinase/stress-activated protein kinase. Chang Gung Med. J. 23:57-72. [PubMed] [Google Scholar]
- 53.Waskiewicz, A. J., and J. A. Cooper. 1995. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr. Opin. Cell Biol. 7:798-805. [DOI] [PubMed] [Google Scholar]
- 54.Webster-Cyriaque, J., and N. Raab-Traub. 1998. Transcription of Epstein-Barr virus latent cycle genes in oral hairy leukoplakia. Virology 248:653-656. [DOI] [PubMed] [Google Scholar]
- 55.Winberg, G., L. Matskova, F. Chen, P. Plant, D. Rotin, G. Gish, R. Ingham, I. Ernberg, and T. Pawson. 2000. Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol. Cell. Biol. 20:8526-8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong, W. Y., L. S. Havarstein, I. M. Morgan, and P. K. Vogt. 1992. c-Jun causes focus formation and anchorage-independent growth in culture but is non-tumorigenic. Oncogene 7:2077-2080. [PubMed] [Google Scholar]
- 57.Xie, H., M. A. Pallero, K. Gupta, P. Chang, M. F. Ware, W. Witke, D. J. Kwiatkowski, D. A. Lauffenburger, J. E. Murphy-Ullrich, and A. Wells. 1998. EGF receptor regulation of cell motility: EGF induces disassembly of focal adhesions independently of the motility-associated PLCgamma signaling pathway. J. Cell Sci. 111:615-624. [DOI] [PubMed] [Google Scholar]