Abstract
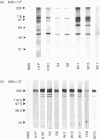
The laboratory-adapted K181 strain of murine cytomegalovirus (MCMV) induces both acute and chronic myocarditis, associated with autoantibodies to cardiac myosin, in susceptible BALB/c mice. However, the K181 MCMV strain has been maintained in the laboratory for many years and may not resemble naturally occurring strains of MCMV in its ability to induce myocarditis. Accordingly, six different isolates of MCMV from wild Mus domesticus were compared with K181 MCMV for their ability to induce myocarditis and autoantibodies to cardiac myosin in BALB/c mice. These isolates were shown to induce acute myocarditis similar to K181 MCMV, with associated focal and diffuse myocardial inflammation. However, the levels of myocarditis induced by the wild isolates during the chronic phase of the disease (days 32-56 post-infection) were low in contrast to the K181 strain. Interestingly, 30% of wild-trapped mice showed histological evidence of myocarditis and all were sero-positive to MCMV. Sera from BALB/c mice infected with wild MCMV isolates and from wild-trapped mice contained antibodies that cross-reacted with MCMV and cardiac myosin (S2 region). The cross-reactive region of MCMV was found to be a 50,000-55,000 MW viral polypeptide. These findings suggest that molecular mimicry may be involved in the pathogenesis of autoimmune myocarditis following infection with both laboratory and wild MCMV strains.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomaeus W. N., O'Donoghue H., Foti D., Lawson C. M., Shellam G. R., Reed W. D. Multiple autoantibodies following cytomegalovirus infection: virus distribution and specificity of autoantibodies. Immunology. 1988 Jul;64(3):397–405. [PMC free article] [PubMed] [Google Scholar]
- Beall J. A., Mitchell G. F. Identification of a particular antigen from a parasite cDNA library using antibodies affinity purified from selected portions of Western blots. J Immunol Methods. 1986 Feb 12;86(2):217–223. doi: 10.1016/0022-1759(86)90456-4. [DOI] [PubMed] [Google Scholar]
- Booth T. W., Scalzo A. A., Carrello C., Lyons P. A., Farrell H. E., Singleton G. R., Shellam G. R. Molecular and biological characterization of new strains of murine cytomegalovirus isolated from wild mice. Arch Virol. 1993;132(1-2):209–220. doi: 10.1007/BF01309855. [DOI] [PubMed] [Google Scholar]
- Chapman A. J., Farrell H. E., Thomas J. A., Papadimitriou J. M., Garlepp M. J., Scalzo A. A., Shellam G. R. A murine cytomegalovirus-neutralizing monoclonal antibody exhibits autoreactivity and induces tissue damage in vivo. Immunology. 1994 Mar;81(3):435–443. [PMC free article] [PubMed] [Google Scholar]
- Chow L. H., Gauntt C. J., McManus B. M. Differential effects of myocarditic variants of Coxsackievirus B3 in inbred mice. A pathologic characterization of heart tissue damage. Lab Invest. 1991 Jan;64(1):55–64. [PubMed] [Google Scholar]
- Craighead J. E., Martin W. B., Huber S. A. Role of CD4+ (helper) T cells in the pathogenesis of murine cytomegalovirus myocarditis. Lab Invest. 1992 Jun;66(6):755–761. [PubMed] [Google Scholar]
- Davies J. M. Molecular mimicry: can epitope mimicry induce autoimmune disease? Immunol Cell Biol. 1997 Apr;75(2):113–126. doi: 10.1038/icb.1997.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell H. E., Shellam G. R. Characterization of neutralizing monoclonal antibodies to murine cytomegalovirus. J Gen Virol. 1990 Mar;71(Pt 3):655–664. doi: 10.1099/0022-1317-71-3-655. [DOI] [PubMed] [Google Scholar]
- Farrell H. E., Shellam G. R. Immunoblot analysis of the antibody response to murine cytomegalovirus in genetically resistant and susceptible mice. J Gen Virol. 1989 Oct;70(Pt 10):2573–2586. doi: 10.1099/0022-1317-70-10-2573. [DOI] [PubMed] [Google Scholar]
- Friman G., Wesslén L., Fohlman J., Karjalainen J., Rolf C. The epidemiology of infectious myocarditis, lymphocytic myocarditis and dilated cardiomyopathy. Eur Heart J. 1995 Dec;16 (Suppl O):36–41. doi: 10.1093/eurheartj/16.suppl_o.36. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Arizpe H. M., Higdon A. L., Rozek M. M., Crawley R., Cunningham M. W. Anti-Coxsackievirus B3 neutralizing antibodies with pathological potential. Eur Heart J. 1991 Aug;12 (Suppl 500):124–129. doi: 10.1093/eurheartj/12.suppl_d.124. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Arizpe H. M., Higdon A. L., Wood H. J., Bowers D. F., Rozek M. M., Crawley R. Molecular mimicry, anti-coxsackievirus B3 neutralizing monoclonal antibodies, and myocarditis. J Immunol. 1995 Mar 15;154(6):2983–2995. [PubMed] [Google Scholar]
- Huber S. A. Autoimmunity in myocarditis: relevance of animal models. Clin Immunol Immunopathol. 1997 May;83(2):93–102. doi: 10.1006/clin.1997.4342. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Cunningham M. W. Streptococcal M protein peptide with similarity to myosin induces CD4+ T cell-dependent myocarditis in MRL/++ mice and induces partial tolerance against coxsakieviral myocarditis. J Immunol. 1996 May 1;156(9):3528–3534. [PubMed] [Google Scholar]
- Kim K. S., Sapienza V. J., Carp R. I., Moon H. M. Analysis of structural polypeptides of purified human cytomegalovirus. J Virol. 1976 Dec;20(3):604–611. doi: 10.1128/jvi.20.3.604-611.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama M., Matsumoto Y., Fujiwara M., Masani F., Izumi T., Shibata A. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol. 1990 Nov;57(2):250–262. doi: 10.1016/0090-1229(90)90039-s. [DOI] [PubMed] [Google Scholar]
- Lauer B., Padberg K., Schultheiss H. P., Strauer B. E. Autoantibodies against human ventricular myosin in sera of patients with acute and chronic myocarditis. J Am Coll Cardiol. 1994 Jan;23(1):146–153. doi: 10.1016/0735-1097(94)90513-4. [DOI] [PubMed] [Google Scholar]
- Lawson C. M., Grundy J. E., Shellam G. R. Antibody responses to murine cytomegalovirus in genetically resistant and susceptible strains of mice. J Gen Virol. 1988 Aug;69(Pt 8):1987–1998. doi: 10.1099/0022-1317-69-8-1987. [DOI] [PubMed] [Google Scholar]
- Lawson C. M., Grundy J. E., Shellam G. R. Delayed-type hypersensitivity responses to murine cytomegalovirus in genetically resistant and susceptible strains of mice. J Gen Virol. 1987 Sep;68(Pt 9):2379–2388. doi: 10.1099/0022-1317-68-9-2379. [DOI] [PubMed] [Google Scholar]
- Lawson C. M., O'Donoghue H. L., Farrell H. E., Shellam G. R., Reed W. D. Murine anti-cytomegalovirus monoclonal antibodies with autoreactivity. Immunology. 1991 Mar;72(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- Lawson C. M., O'Donoghue H. L., Reed W. D. Mouse cytomegalovirus infection induces antibodies which cross-react with virus and cardiac myosin: a model for the study of molecular mimicry in the pathogenesis of viral myocarditis. Immunology. 1992 Mar;75(3):513–519. [PMC free article] [PubMed] [Google Scholar]
- Lawson C. M., O'Donoghue H., Bartholomaeus W. N., Reed W. D. Genetic control of mouse cytomegalovirus-induced myocarditis. Immunology. 1990 Jan;69(1):20–26. [PMC free article] [PubMed] [Google Scholar]
- Lawson C. M., O'Donoghue H., Reed W. D. The role of T cells in mouse cytomegalovirus myocarditis. Immunology. 1989 May;67(1):132–134. [PMC free article] [PubMed] [Google Scholar]
- Leach C. T., Detels R., Hennessey K., Liu Z., Visscher B. R., Dudley J. P., Cherry J. D. A longitudinal study of cytomegalovirus infection in human immunodeficiency virus type 1-seropositive homosexual men: molecular epidemiology and association with disease progression. J Infect Dis. 1994 Aug;170(2):293–298. doi: 10.1093/infdis/170.2.293. [DOI] [PubMed] [Google Scholar]
- Liao L., Sindhwani R., Leinwand L., Diamond B., Factor S. Cardiac alpha-myosin heavy chains differ in their induction of myocarditis. Identification of pathogenic epitopes. J Clin Invest. 1993 Dec;92(6):2877–2882. doi: 10.1172/JCI116909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch B., Schönian U., Crombach M., Wendl I., Bethge C., Herzum M., Klein H. H. Cytomegalovirus associated inflammatory heart muscle disease. Scand J Infect Dis Suppl. 1993;88:135–148. [PubMed] [Google Scholar]
- Neumann D. A., Burek C. L., Baughman K. L., Rose N. R., Herskowitz A. Circulating heart-reactive antibodies in patients with myocarditis or cardiomyopathy. J Am Coll Cardiol. 1990 Nov;16(6):839–846. doi: 10.1016/s0735-1097(10)80331-6. [DOI] [PubMed] [Google Scholar]
- O'Donoghue H. L., Lawson C. M., Reed W. D. Autoantibodies to cardiac myosin in mouse cytomegalovirus myocarditis. Immunology. 1990 Sep;71(1):20–28. [PMC free article] [PubMed] [Google Scholar]
- Rawlinson W. D., Farrell H. E., Barrell B. G. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996 Dec;70(12):8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N. R., Herskowitz A., Neumann D. A. Autoimmunity in myocarditis: models and mechanisms. Clin Immunol Immunopathol. 1993 Aug;68(2):95–99. doi: 10.1006/clin.1993.1102. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Singleton G. R., Hansen G. M., Shellam G. A serologic survey for viruses and Mycoplasma pulmonis among wild house mice (Mus domesticus) in southeastern Australia. J Wildl Dis. 1993 Apr;29(2):219–229. doi: 10.7589/0090-3558-29.2.219. [DOI] [PubMed] [Google Scholar]
- Smith S. C., Allen P. M. The role of T cells in myosin-induced autoimmune myocarditis. Clin Immunol Immunopathol. 1993 Aug;68(2):100–106. doi: 10.1006/clin.1993.1103. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J., Sissons J. G., Borysiewicz L. K., Sinclair J. H. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991 Sep;72(Pt 9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- Tracy S., Chapman N. M., Romero J., Ramsingh A. I. Genetics of coxsackievirus B cardiovirulence and inflammatory heart muscle disease. Trends Microbiol. 1996 May;4(5):175–179. doi: 10.1016/0966-842x(96)10026-3. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- von Herrath M. G., Oldstone M. B. Role of viruses in the loss of tolerance to self-antigens and in autoimmune diseases. Trends Microbiol. 1995 Nov;3(11):424–430. doi: 10.1016/s0966-842x(00)88995-7. [DOI] [PubMed] [Google Scholar]