Abstract
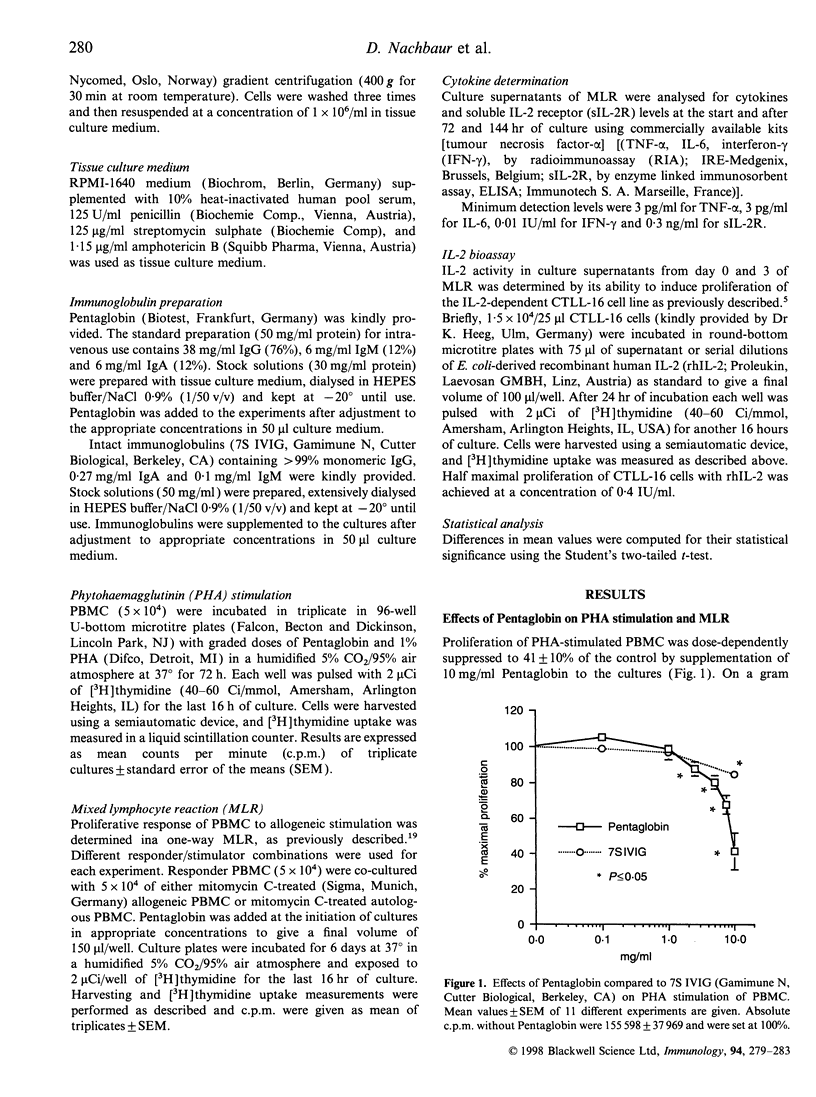
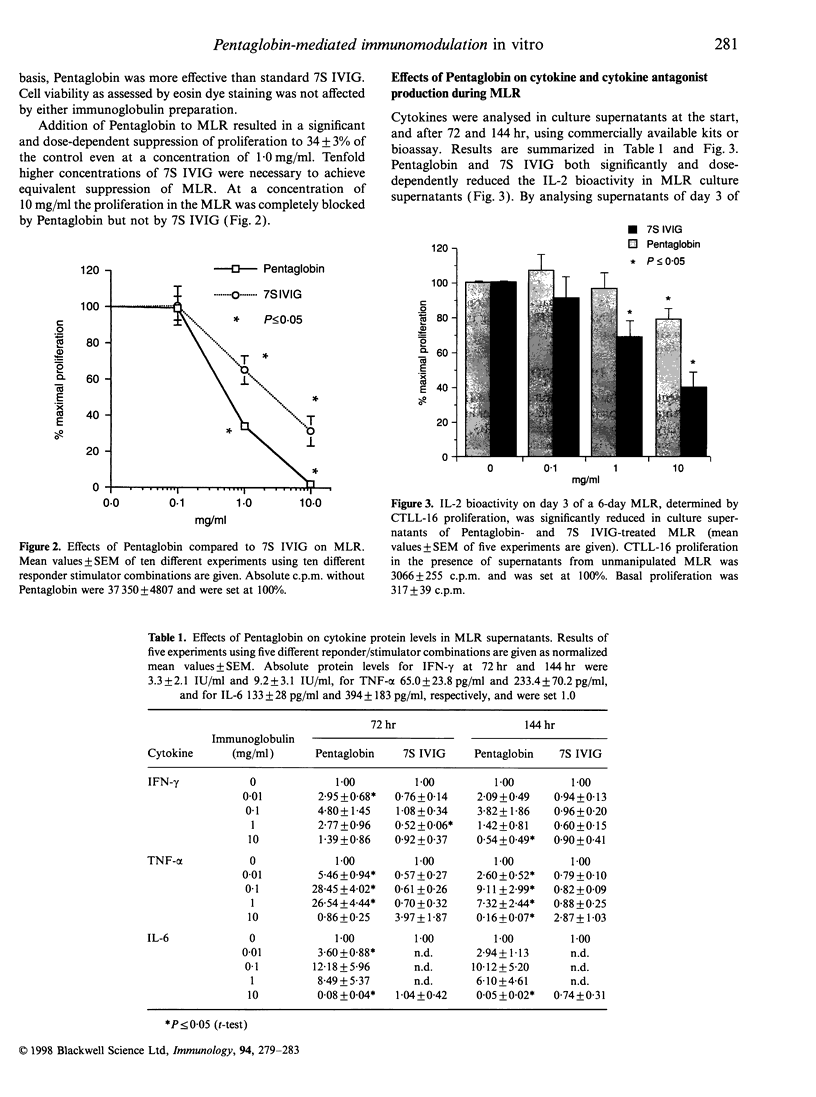
The mode of action of intravenous immunoglobulins (IVIG) in autoimmune and immunoregulatory disorders is still poorly understood. In vitro, direct effects of IVIG on cytokine release and on cytokine receptors have been described, as well as naturally occurring, neutralizing anticytokine antibodies. The aim of our study was to investigate whether the enrichment in IgM and IgA would have any impact on the in vitro immunomodilatory capacity of IVIG. The preparation tested (Pentaglobin) contains 76% IgG and 12% IgM and IgA, respectively. We could demonstrate a significant inhibition of alloantigen-induced proliferation in the mixed lymphocyte reaction (MLR) even at a Pentaglobin concentration of 1.0 mg/ml. About 10-fold higher concentrations of standard 7S IVIG containing only trace amounts of IgM and IgA were necessary to achieve equivalent suppression of the alloimmune response. Similarly, phytohaemagglutinin (PHA)-induced lymphocyte proliferation was more effectively inhibited by Pentaglobin than by standard 7S IVIG. Cytokine analyses in culture supernatants of MLR provide evidence that Pentaglobin not only modulates interleukin-2 (IL-2), which has already been observed with standard 7S IVIG, but, moreover, modulates interferon-gamma production with a subsequent impact on monocyte-derived tumour necrosis factor-alpha and IL-6 release. Based on these results we conclude that in vitro the IgM- and IgA-enriched Pentaglobin has a more potent immunomodulatory capacity than conventionally used standard 7S IVIG.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Horiuchi A., Miyake M., Kimura S. Anti-cytokine nature of natural human immunoglobulin: one possible mechanism of the clinical effect of intravenous immunoglobulin therapy. Immunol Rev. 1994 Jun;139:5–19. doi: 10.1111/j.1600-065x.1994.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Amran D., Renz H., Lack G., Bradley K., Gelfand E. W. Suppression of cytokine-dependent human T-cell proliferation by intravenous immunoglobulin. Clin Immunol Immunopathol. 1994 Nov;73(2):180–186. doi: 10.1006/clin.1994.1186. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Is there a role for interleukin-1 blockade in intravenous immunoglobulin therapy? Immunol Rev. 1994 Jun;139:173–188. doi: 10.1111/j.1600-065x.1994.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Dwyer J. M. Manipulating the immune system with immune globulin. N Engl J Med. 1992 Jan 9;326(2):107–116. doi: 10.1056/NEJM199201093260206. [DOI] [PubMed] [Google Scholar]
- Holler E., Kolb H. J., Möller A., Kempeni J., Liesenfeld S., Pechumer H., Lehmacher W., Ruckdeschel G., Gleixner B., Riedner C. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990 Feb 15;75(4):1011–1016. [PubMed] [Google Scholar]
- Huber C., Fink U., Leibold W., Schmalzl F., Peterson P. A., Klareskog L., Braunsteiner H. The role of adherent HLA-DR+ mononuclear cells in autologous and allogeneic MLR. J Immunol. 1981 Aug;127(2):726–731. [PubMed] [Google Scholar]
- Hurez V., Kazatchkine M. D., Vassilev T., Ramanathan S., Pashov A., Basuyaux B., de Kozak Y., Bellon B., Kaveri S. V. Pooled normal human polyspecific IgM contains neutralizing anti-idiotypes to IgG autoantibodies of autoimmune patients and protects from experimental autoimmune disease. Blood. 1997 Nov 15;90(10):4004–4013. [PubMed] [Google Scholar]
- Imbach P., Barandun S., d'Apuzzo V., Baumgartner C., Hirt A., Morell A., Rossi E., Schöni M., Vest M., Wagner H. P. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981 Jun 6;1(8232):1228–1231. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- Kaveri S. V., Dietrich G., Hurez V., Kazatchkine M. D. Intravenous immunoglobulins (IVIg) in the treatment of autoimmune diseases. Clin Exp Immunol. 1991 Nov;86(2):192–198. doi: 10.1111/j.1365-2249.1991.tb05794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaesson S., Ringdén O., Ljungman P., Aschan J., Hägglund H., Winiarski J. Does high-dose intravenous immune globulin treatment after bone marrow transplantation increase mortality in veno-occlusive disease of the liver? Transplantation. 1995 Dec 15;60(11):1225–1230. [PubMed] [Google Scholar]
- Klingemann H. G., Barnett M. J., Reece D. E., Shepherd J. D., Phillips G. L. Use of an immunoglobulin preparation enriched for IgM (Pentaglobin) for the treatment of acute graft-versus-host disease. Bone Marrow Transplant. 1990 Sep;6(3):199–202. [PubMed] [Google Scholar]
- Nachbaur D., Herold M., Eibl B., Glassl H., Schwaighofer H., Huber C., Gächter A., Pichl M., Niederwieser D. A comparative study of the in vitro immunomodulatory activity of human intact immunoglobulin (7S IVIG), F(ab')2 fragments (5S IVIG) and Fc fragments. Evidence for post-transcriptional IL-2 modulation. Immunology. 1997 Feb;90(2):212–218. doi: 10.1046/j.1365-2567.1997.d01-2148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburger J. W., Takahashi M., Burns J. C., Beiser A. S., Chung K. J., Duffy C. E., Glode M. P., Mason W. H., Reddy V., Sanders S. P. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986 Aug 7;315(6):341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- Niederwieser D., Herold M., Woloszczuk W., Aulitzky W., Meister B., Tilg H., Gastl G., Bowden R., Huber C. Endogenous IFN-gamma during human bone marrow transplantation. Analysis of serum levels of interferon and interferon-dependent secondary messages. Transplantation. 1990 Oct;50(4):620–625. doi: 10.1097/00007890-199010000-00019. [DOI] [PubMed] [Google Scholar]
- Peraldi M. N., Akposso K., Haymann J. P., Flahaut A., Marlin C., Rondeau E., Sraer J. D. Long-term benefit of intravenous immunoglobulins in cadaveric kidney retransplantation. Transplantation. 1996 Dec 15;62(11):1670–1673. doi: 10.1097/00007890-199612150-00024. [DOI] [PubMed] [Google Scholar]
- Poynton C. H., Jackson S., Fegan C., Barnes R. A., Whittaker J. A. Use of IgM enriched intravenous immunoglobulin (Pentaglobin) in bone marrow transplantation. Bone Marrow Transplant. 1992 Jun;9(6):451–457. [PubMed] [Google Scholar]
- Ross C., Svenson M., Hansen M. B., Vejlsgaard G. L., Bendtzen K. High avidity IFN-neutralizing antibodies in pharmaceutically prepared human IgG. J Clin Invest. 1995 May;95(5):1974–1978. doi: 10.1172/JCI117881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanz U., Hügle T., Gmür J. Additional inhibitory effects of intravenous immunoglobulins in combination with cyclosporine A on human T lymphocyte alloproliferative response in vitro. Transplantation. 1996 Jun 27;61(12):1736–1740. doi: 10.1097/00007890-199606270-00013. [DOI] [PubMed] [Google Scholar]
- Schwaighofer H., Oberhuber G., Hebart H., Einsele H., Herold M., Nachbaur D., Eibl B., Tilg H., Kropshofer G., Ferrara J. L. Endogenous interleukin 1 receptor antagonist during human bone marrow transplantation: increased levels during graft-versus-host disease, during infectious complications, and after immunoglobulin therapy. Transplantation. 1997 Jan 15;63(1):52–56. doi: 10.1097/00007890-199701150-00010. [DOI] [PubMed] [Google Scholar]
- Shulman H. M., Hinterberger W. Hepatic veno-occlusive disease--liver toxicity syndrome after bone marrow transplantation. Bone Marrow Transplant. 1992 Sep;10(3):197–214. [PubMed] [Google Scholar]
- Svenson M., Hansen M. B., Bendtzen K. Binding of cytokines to pharmaceutically prepared human immunoglobulin. J Clin Invest. 1993 Nov;92(5):2533–2539. doi: 10.1172/JCI116862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toungouz M., Denys C., Dupont E. Blockade of proliferation and tumor necrosis factor-alpha production occurring during mixed lymphocyte reaction by interferon-gamma-specific natural antibodies contained in intravenous immunoglobulins. Transplantation. 1996 Nov 15;62(9):1292–1296. doi: 10.1097/00007890-199611150-00020. [DOI] [PubMed] [Google Scholar]