Abstract
Vectors derived from lentiviruses provide a promising gene delivery system. We examined the in vivo gene transfer efficiency and tissue or cell tropism of a feline immunodeficiency virus (FIV)-based lentiviral vector pseudotyped with the glycoproteins from Ross River Virus (RRV). RRV glycoproteins were efficiently incorporated into FIV virions, generating preparations of FIV vector, which after concentration attain titers up to 1.5 × 108 TU/ml. After systemic administration, RRV-pseudotyped FIV vectors (RRV/FIV) predominantly transduced the liver of recipient mice. Transduction efficiency in the liver with the RRV/FIV was ca. 20-fold higher than that achieved with the vesicular stomatitis virus G protein (VSV-G) pseudotype. Moreover, in comparison to VSV-G, the RRV glycoproteins caused less cytotoxicity, as determined from the levels of glutamic pyruvic transaminase and glutamic oxalacetic transaminase in serum. Although hepatocytes were the main liver cell type transduced, nonhepatocytes (mainly Kupffer cells) were also transduced. The percentages of the transduced nonhepatocytes were comparable between RRV and VSV-G pseudotypes and did not correlate with the production of antibody against the transgene product. After injection into brain, RRV/FIV preferentially transduced neuroglial cells (astrocytes and oligodendrocytes). In contrast to the VSV-G protein that targets predominantly neurons, <10% of the brain cells transduced with the RRV pseudotyped vector were neurons. Finally, the gene transfer efficiencies of RRV/FIV after direct application to skeletal muscle or airway were also examined and, although transgene-expressing cells were detected, their proportions were low. Our data support the utility of RRV glycoprotein-pseudotyped FIV lentiviral vectors for hepatocyte- and neuroglia-related disease applications.
Gene transfer provides a potentially powerful tool for the treatment of a wide variety of diseases. Viral vectors derived from lentiviruses, such as human immunodeficiency virus (HIV) (25, 31, 36), feline immunodeficiency virus (FIV) (24, 37, 51), and others (3, 33) have been developed and utilized in vitro and in vivo to transfer genes of interest to somatic cells. Lentivirus-based vectors are attractive gene delivery vehicles because they can transduce both dividing and nondividing cells, resulting in stable integration and long-term expression of the transgene.
Lentivirus-mediated gene transfer begins with the attachment of the virion to a specific cell surface receptor, followed by virus-cell fusion and penetration of the nucleocapsid into the cell, reverse transcription of the viral RNA genome, integration of the viral DNA, and ultimately expression of the transgene (10). The attachment of the virion to the host cell receptor is the first step in the entire gene delivery process and a crucial factor in determining vector tropism and the range of target tissues or cell types. This viral attachment is mediated by specific interactions between the envelope glycoprotein (Env) on the virion and one or more surface receptor molecules on the target cell. If this receptor molecule is absent (as when its expression is specific for certain cell types) or is variant in the binding region (such as in species other than the natural host), gene transfer cannot occur (10). By replacing the original Env protein with other viral glycoproteins, a process termed “pseudotyping,” one can alter the host range of the vectors, which may result in increased transduction efficiency of target cells.
Many examples of pseudotyping exist in the literature (7, 14, 18, 27-29, 40, 41, 44). Vesicular stomatitis virus G protein (VSV-G) and the amphotropic envelope protein are the two most commonly used viral glycoproteins in current lentivirus-based gene transfer. However, both Env glycoproteins have limitations for potential clinical use. For example, VSV-G is cytotoxic (35) and may be inactivated by human serum (13), and the amphotropic envelope is fragile and does not tolerate centrifugation concentration methods as well as does VSV-G (7). Moreover, receptor abundance or localization may further limit the utility of these two Env glycoproteins (47, 50, 51).
In an effort to increase the in vivo gene transfer efficiency of lentiviral vectors in specific target cells/tissues, we pseudotyped the FIV-based nonprimate lentiviral vector with the glycoproteins from Ross River Virus (RRV). RRV is an arthropod-borne alphavirus and causes epidemic polyarthritis, myalgia, arthralgia, and lethargy in humans (46). RRV infects both invertebrates and vertebrates, including mosquitoes, reptiles, birds, and mammals. The virus can replicate within neurons and glial cells, striated and smooth muscle cells, cells of lymphoid origin, synovial cells, and many others (46). Given the extremely broad host or cell ranges of the wild-type RRV, we reasoned that pseudotyping the FIV vector with RRV glycoproteins would result in improved gene transfer to target cells and extend the range of tissues that can be transduced.
In the current study, we examined the in vivo gene transfer efficiency and cell-tissue tropism of the RRV-pseudotyped FIV vector in mice. The RRV/FIV vector encoding a β-galactosidase reporter gene was (i) injected intravenously for systemic gene delivery, (ii) injected into brain parenchyma, (iii) injected intramuscularly, and (iv) instilled into the lung for gene delivery to airway epithelial cells. Our data indicate that the RRV pseudotyped FIV vector transduces hepatocytes more efficiently than the VSV-G Env with less hepatotoxicity. Moreover, the RRV glycoproteins exhibit cell tropism in the central nervous system that is distinct from the VSV-G pseudotype. These findings demonstrate potential applications of using RRV pseudotyped FIV vectors for disease therapies.
MATERIALS AND METHODS
FIV vector constructs and particle production.
RRV- or VSV-G-pseudotyped FIV vector (VSV-G/FIV) particles were produced by using a three-plasmid expression system as described previously (24, 42, 43). The packaging plasmid, pCFIVΔorfΔvif, contains a deletion in the env gene and mutations in vif and orf2. The 5′ and 3′ long terminal repeats were replaced with the cytomegalovirus (CMV) promoter-enhancer and the simian virus 40 polyadenylation signal, respectively. The vector plasmid is based on the pVETL backbone, which contains the CMV promoter-enhancer in place of the 5′ U3, a truncated gag, and a multiple cloning site. An expression cassette containing the cDNA for cytoplasm-localized β-galactosidase or eGFP, directed by the CMV promoter-enhancer, was ligated into the multiple cloning site of pVETL. The VSV-G envelope plasmid, pCMV-G, encodes the VSV envelope glycoprotein. The pRRV-E2E1 plasmid encodes the full-length RRV envelope glycoprotein, E3-E2-6K-E1, which is processed proteolytically into the individual subunits (41).
Vector particles were generated by transient transfection of 293T cells with packaging, envelope, and vector plasmids, followed by the collection of supernatants and particle concentration by centrifugation. For each preparation, we routinely centrifuge 750 ml of culture supernatant overnight at 7,400 × g and resuspend in 3 ml of lactose buffer (42, 50). Transduction titers before and after concentration were determined by measurement of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)-positive cells in transduced HT-1080 target cells and were expressed as transducing units (TU)/milliliter. β-Galactosidase activities in concentrated vector preparations were measured by the Galacto-Light chemiluminescent reporter assay (Tropix, Bedford, Mass.). Immunological detection of RRV E2 and E1 glycoproteins in the FIV vector preparations was performed as described previously (41). The viral p24 levels in the vector preparations used for our in vivo studies were assayed by enzyme-linked immunosorbent assay (ELISA) (24, 48). The capture antibody was PAK1-2B2, and the detecting antibody was bioinylated PAK3-2C1 (Custom Monoclonal Antibodies International, West Sacramento, Calif.). An FIV preparation with a known p24 concentration was used as a standard.
In vivo gene transfer protocols.
All mice used in the present study were housed at the University of Iowa Animal Care Facilities, and all animal procedures were approved by the Animal Care and Use Review Committee at the University of Iowa. Adult (5 to 8 weeks old) C57BL/6 or BALB/c mice (Jackson Laboratories, Bar Harbor, Maine) were used. For intravenous gene transfer experiments, C57BL/6 mice with factor VIII deficiency were used (43).
For systemic vector delivery, mice received either the RRV/FIV vector or VSV-G/FIV vector intravenously via the tail vein. Both vectors were delivered as a single bolus in two experiments, and in a third experiment injections were repeated the next day to double the volume of administered vector. The volume of each injection was 0.4 ml, and the administered vector doses are indicated in the text. On days 1 and 7 postinjection, we obtained blood samples from the retro-orbital plexus and assayed serum samples for the levels of glutamic oxalacetic transaminase (SGOT) and glutamic pyruvic transaminase (SGPT). At 3 weeks postinjection, we collected blood samples for measurement of antibody production against viral p24 antigen and β-galactosidase. The mice were then sacrificed and perfused with cold phosphate-buffered saline (PBS). Samples of liver, spleen, kidney, lung, heart, and skeletal muscles (triceps) were harvested for X-Gal staining, Galacto-Light β-galactosidase enzyme activity assay, and immunohistochemistry as described below.
For vector delivery to the brain, 6- to 8-week-old adult male C57BL/6 mice were used. Direct injections of vector were performed as previously described (19). Mice were anesthetized with ketamine-xylazine (ketamine, 100 mg/kg; xylazine, 10 mg/kg). A total of 5 μl of RRV-pseudotyped vector containing 5 × 105 TU was stereotactically injected into the striatum at coordinates +2 mm lateral and 0.4 mm rostral to the bregma and 3 mm deep by using a 26-gauge Hamilton syringe driven by a microinjector (Micro 1; World Precision Instruments, Sarasota, Fla.) at 0.5 μl per min. At 3 weeks postinjection, mice were sacrificed and perfused with 2% paraformaldehyde in PBS. The brains were postfixed overnight at 4°C and cryoprotected in 30% sucrose-PBS for 48 h at 4°C. The hemispheres were separated and blocked in O.C.T. (Sakura Finetek USA, Torrance, Calif.) by freezing in a dry ice-ethanol bath. Parasagittal cryosections (10 μm) were cut and placed on slides. Slides were stained histochemically for β-galactosidase activity (i.e., X-Gal staining) and counterstained with neutral red or were dually stained with antibodies for immunofluorescent confocal analysis.
For direct injections into the quadriceps skeletal muscle, mice were anesthetized with ketamine-xylazine and the surgical area was shaved and prepped with iodine. A small incision was made through the skin over the quadriceps, and 30 μl of RRV (9 × 105 TU)- or VSV-G (2.7 × 107 TU)-pseudotyped vector was injected into the muscle, the needle was slowly withdrawn, and the incision was sutured with 4-0 nylon. Mice received vector bilaterally, and at 3 weeks postinjection the quadriceps were dissected. The right quadriceps were frozen at −80°C until use in the Galacto-Light assay. The left quadriceps were covered in O.C.T. and frozen in liquid N2-cooled isopentane. Cross sections (10 μm) were cut and placed on slides. Slides were brought to room temperature, fixed for 10 min in 0.5% glutaraldehyde-PBS, washed in PBS, stained histochemically for β-galactosidase activity (X-Gal staining), and counterstained with neutral red.
For in vivo pulmonary gene delivery, mice received instillation of vector preparations, as described previously (39). Briefly, mice were lightly anesthetized by halothane inhalation. Fifty microliters (∼2.5 × 107 TU) of viral vector was instilled dropwise intranasally and allowed to be inhaled into the lungs of five C57BL/6 mice. An additional 50 μl of vector was instilled into each mouse the following day. At 2 weeks after vector instillation, mice were euthanized and perfused through the right ventricle with 2% paraformaldehyde-PBS. The lungs were then removed and assayed for β-galactosidase expression.
Measurement of SGOT and SGPT levels and p24 and β-galactosidase antibody production.
SGOT and SGPT levels were measured by using a transaminase assay kit (505-0P; Sigma, St. Louis, Mo.) according to the manufacturer's instructions and with a minor modification. The volumes of serum, reagents, and standard solution were scaled down by 40-fold. The normal ranges of SGOT and SGPT in mice are <28 U/ml (borderline range, 28 to 40 U/ml) and <21 U/ml (borderline range, 21 to 35 U/ml), respectively. We determined serum p24/ml-specific or β-galactosidase-specific immunoglobulin G (IgG) and IgM responses by ELISA. Briefly, the ELISA plates were coated with 5 μg of recombinant p24 (IDEXX Laboratories, Inc., Westbrook, Maine) or 10 μg of β-galactosidase (Sigma)/ml. After incubation with mouse sera, the plates were probed with alkaline phosphatase-conjugated goat anti-mouse IgG and IgM (Southern Biotechnology Associates, Inc., Birmingham, Ala.) as secondary reagents. Antibody titers were established by CA-Cricket Graph software as the serum dilution that reached preinjection absorbance levels (A405 ∼ 0.134), assuming linear extrapolation.
Determination of β-galactosidase expression.
For X-Gal staining of liver after intravenous vector injection, lobes were fixed in 2% paraformaldehyde-PBS overnight and then stained with X-Gal overnight at 4°C. The overall expression of β-galactosidase was first examined by stereo microscopy. The X-Gal-stained tissue was then embedded in paraffin, and 5-μm sections were cut at 50-μm intervals and counterstained with nuclear fast red or hematoxylin and eosin for quantification and histological examination (51). For X-Gal staining of brain and muscle sections, 10-μm sections on slides were incubated in X-Gal for 6 h at 37°C, washed in PBS, and counterstained with neutral red. For X-Gal staining of lung, the lungs were removed, inflated with and submersed in 2% paraformaldehyde-PBS, and allowed to fix for 4 h at 4°C. After fixation, the lungs were washed with PBS and inflated with X-Gal solution. The lungs were submersed in additional X-Gal and incubated overnight at 37°C. After X-Gal staining, the lungs were washed with PBS and paraffin embedded by a standard protocol, and 10-μm sections were collected. Sections were counterstained with nuclear fast red.
The Galacto-Light chemiluminescent reporter assay was used to quantify β-galactosidase activity in tissues and in the vector preparations. Briefly, tissues were homogenized, and endogenous tissue β-galactosidase was eliminated by heat inactivation in conjunction with protease inhibitors according to the manufacturer's instructions. Tissue extracts were then assayed for β-galactosidase activities as well as for total protein concentrations. For the measurement of β-galactosidase activity in the vector preparations, 18 μl of lysis buffer (included in the assay kit) was added to 2 μl of vector sample and the whole lysate was used for the assay. β-Galactosidase concentrations were determined by interpolation off a standard curve established from twofold serial dilutions of purified β-galactosidase (Sigma). The levels of β-galactosidase expression are represented as nanograms of β-galactosidase per milligram of protein (after intravenous vector injection) or as total picograms of β-galactosidase (after injection into the quadriceps).
Immunostaining.
To assess the extent of Kupffer cell transduction in the liver by RRV- or VSV-G-pseudotyped FIV vectors, colocalization of β-galactosidase and Kupffer cell-specific marker (Ly-71) was performed on frozen mouse liver sections, as described previously (57). Briefly, 5-μm sections were incubated with biotin-conjugated rat monoclonal antibody to mouse F4/80 (1/100; macrophage Ly-71; Caltag Laboratories, Burlingame, Calif.), followed by incubation with Texas red-conjugated avidin (1/150; Caltag Laboratories). After three washes in 1.5% rabbit serum-PBS, the slides were stained with fluorescein isothiocyanate- conjugated rabbit anti-β-galactosidase antibody (1/100) (Research Diagnostics, Inc., Flanders, N.J.). Sections were then visualized by fluorescent microscopy under Texas red- and fluorescein isothiocyanate-specific filters. Colocalization was assessed as with brain, described below.
To determine the cell types transduced after intrastriatal injections of RRV pseudotyped FIV, 10-μm brain sections were dually stained for β-galactosidase and glial fibrillary acidic protein (GFAP, a type II astrocyte-specific intermediate filament), for β-galactosidase and NeuN (a nuclear antigen common to most neurons), or for β-galactosidase and 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase; an oligodendrocyte-myelin specific marker) and analyzed by confocal fluorescence microscopy. The antibodies used were polyclonal rabbit anti-β-galactosidase (Biodesign International, Saco, Maine), Cy3-conjugated mouse monoclonal anti-GFAP (Sigma), mouse monoclonal anti-NeuN (Chemicon International, Temecula, Calif.), mouse monoclonal anti-CNPase (Sigma), Alexa 488-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, Oreg.), and lissamine-rhodamine-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, Pa.). Sections (10 μm) were stained as previously described (42). Briefly, sections were blocked with 10% normal goat serum and 0.1% Triton X-100 in PBS for 1 h at room temperature and then incubated with primary antibodies overnight at 4°C in PBS with 3% bovine serum albumin and 0.1% Triton X-100. The sections were then washed, incubated with secondary antibodies for 2 h at room temperature, washed, and coverslipped with gel mount. Using confocal microscopy, images from 0.3-μm-thick Z series were collected. Red and green channel images were merged, and areas of colocalization of β-galactosidase signal (green) and cell type-specific marker (red) appear yellow.
RESULTS
RRV envelope glycoproteins incorporate efficiently into recombinant FIV virions, generating high titers of FIV viral particles. To generate RRV glycoprotein-pseudotyped recombinant FIV, 293T cells were transiently transfected with pCFIVΔorfΔvif packaging plasmid, pVETLCMV-β-Gal vector plasmid, and pRRV-E2E1 envelope plasmid. Recombinant FIV particles present in the supernatant medium of the transfected cells were harvested at 12, 24, and 48 h posttransfection and concentrated by centrifugation, and the titers were determined on HT1080 cells. Prior to concentration, supernatants had titers of 5 × 104 to 5 × 105 TU/ml on HT1080 target cells. We found that RRV/FIV was very stable, with almost no loss in transduction units after concentration (vector recovery ranging from 85 to 100%), indicating that the RRV envelope withstands centrifugation. With this centrifugation method, we produced concentrated preparations of RRV/FIV with titers ranging from 2 × 107 to 1.5 × 108 TU/ml. The titer of concentrated RRV-pseudotyped FIV particles is comparable to titers typically obtained by using the VSV-G Env (24, 43, 51).
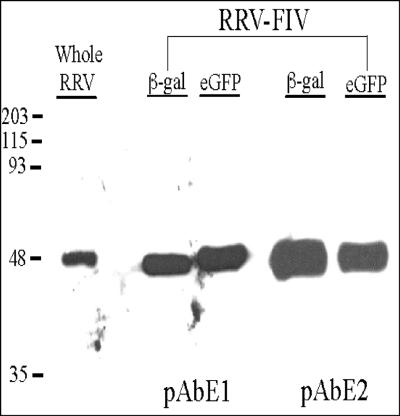
Immunoblotting with antibodies against the RRV glycoproteins E2 and E1 confirmed the expression of both glycoproteins in the recombinant FIV particles (Fig. 1). Both proteins showed mobility identical to the mature E2 and E1 glycoproteins found in the wild-type RRV viruses. These data demonstrate that the RRV envelope glycoproteins are efficiently incorporated into FIV virions and generate titers sufficient for in vivo gene transfer.
FIG. 1.
Immunoblot analysis of RRV E2E1-pseudotyped FIV. Purified wild-type RRV, RRV/FIVβ-gal, and RRV/FIVeGFP were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with polycolonal rabbit antibodies to RRV E2 (pAbE2) and RRV E1 (pAbE1). Immunoblotting was conducted as described previously (41).
RRV-pseudotyped FIV vectors transduce hepatocytes more efficiently than VSV-G pseudotype with reduced side effects.
To determine the in vivo gene transfer efficiency of RRV glycoprotein-pseudotyped FIV vector, as well as its tissue tropism, mice were intravenously injected with lactose buffer control or RRV/FIV. At 3 weeks postinfusion, the recipient mice were sacrificed, and samples of liver, spleen, kidney, lung, heart, and skeletal muscle were harvested and assayed for β-galactosidase activity. As shown in Table 1, the predominant tissue transduced after intravenous injection of RRV-pseudotyped FIV vector was the liver. The β-galactosidase levels, represented as nanograms of enzyme activity per milligram of total protein, were more than 100 times higher in the liver than in other tissues.
TABLE 1.
β-Galactosidase activities in tissue lysatesa
| Tissue | β-Galactosidase level (ng/mg of protein)
|
|
|---|---|---|
| Control | RRV/FIV | |
| Liver | 0.05 ± 0.02 | 13.1 ± 5.52 |
| Spleen | NDb | 0.10 ± 0.05 |
| Kidney | 0.05 ± 0.01 | 0.10 ± 0.03 |
| Lung | ND | 0.12 ± 0.10 |
| Heart | ND | 0.12 ± 0.04 |
| Muscle | ND | 0.11 ± 0.08 |
Systemic administration of RRV/FIV predominantly transduces the liver. Mice were intravenously injected via tail vein with lactose buffer control (n = 5) or 3 × 107 TU of RRV/FIV vector (n = 5). At 3 weeks postinjection, the mice were sacrificed, and the liver, spleen, kidney, lung, heart, and muscle were harvested and assayed for β-galactosidase activities using Galacto-Light. A whole-liver extract has a total of 366.24 ± 26.21 mg of protein, as measured by Lowry assay. Each mouse received 0.4 ml of RRV/FIV vector preparation containing β-galactosidase activity at 2.12 ± 0.05 μg/ml.
ND, not detected (below limits of sensitivity of assay).
To address the possibility that β-galactosidase activity in the liver could be due to pseudotransduction (protein transfer) rather than transgene expression, we measured β-galactosidase levels in the concentrated RRV/FIV vector preparations (2.12 ± 0.05 μg/ml) and quantified the total protein content in a whole liver extracts (366.24 ± 26.21 mg). We injected 0.4 ml of RRV/FIV, which contained a total of ∼0.85 μg of β-galactosidase activity. However, at 3 weeks postinjection the total β-galactosidase activity in the liver was ∼4.8 μg. These data suggest that if all of the β-galactosidase present in the vector preparation reached the liver and remained stable for 3 weeks, it could only account for 18% of the activity detected in the liver. Thus, >80% of the β-galactosidase activity in the liver results from transgene expression.
Similar to RRV/FIV, intravenous injection of VSV-G-pseudotyped FIV predominantly transduces the liver (43; unpublished data). We therefore wished to examine and compare the liver gene transfer efficiency after systemic administration of RRV/FIV or VSV-G/FIV vectors. Mice were injected via the tail vein with lactose buffer, RRV/FIV vector, or VSV-G/FIV vector. Overall tissue expression of β-galactosidase in the X-Gal-stained liver was first examined by stereo microscopy. All mice receiving the RRV/FIV vector expressed significant levels of β-galactosidase (12 mice in total). In contrast, 11 of 14 mice receiving VSV-G/FIV vector expressed β-galactosidase. Moreover, as shown in Fig. 2A to C, the liver was transduced to a much greater extent in the mice receiving the RRV/FIV vector than in those receiving equivalent or higher titers of VSV-G-pseudotyped vector. The β-galactosidase expression was uniformly distributed throughout the liver in the mice receiving the RRV/FIV vector.
FIG. 2.
RRV/FIV transduces the liver more efficiently than VSV-G/FIV with reduced cytotoxicity. Mice were intravenously injected via the tail vein with a single bolus of control lactose buffer (A), 10 × 107 TU of RRV/FIV vector (B), or 35 × 107 TU of VSV-G/FIV vector (C). At 24 h postinjection, the mice were bled, and the blood samples were collected and assayed for SGOT and SGPT (four mice per treatment group). At 3 weeks postinjection, the mice were sacrificed and the liver was stained with X-Gal and examined under stereo microscopy (A to C). (D) Summary of β-galactosidase expression levels in mice receiving two different doses of RRV-pseudotyped FIV vector (3 × 107 TU/mouse, n = 5; 10 × 107 TU/mouse, n = 7). (E) Summary of β-galactosidase expression levels in mice receiving three different doses of VSV-G-pseudotyped FIV vector (7.2 × 107 TU/mouse, n = 3; 35 × 107 TU/mouse, n = 8; 68 × 107 TU/mouse, n = 3). (F) SGOT (left) and SGPT (right) levels from recipient mice on day 1 postinjection. The levels of SGOT and SGPT from the recipient mice of RRV/FIV vector were not different from those of buffer control recipients and were within the normal range. In contrast, the mice receiving the VSV-G/FIV vector had significantly higher levels of SGOT and SGPT than both control mice and RRV/FIV recipient mice on day 1 postinjection (✽, P < 0.05 [two-tailed Student t test]). On day 7, the levels of SGOT and SGPT in these mice returned to normal range. The results of one of three representative experiments is shown, with a total of ≥12 mice per treatment group.
We next quantified the number of β-galactosidase-positive cells in the livers of mice receiving either RRV/FIV or VSV-G/FIV vector. The X-Gal-stained liver tissues were embedded in paraffin. Serial sections were cut every 50 μm, counterstained with nuclear fast red, and examined for β-galactosidase-positive cells. At least 20 representative fields were examined (at a magnification of ×20) per animal to determine the total frequency of β-galactosidase-positive cells. The levels of expression are represented as β-galactosidase-positive cells per ×20 field. Each ×20 field has a total cell number of 429 ± 10 (mean ± the standard error, n = 253 ×20 fields). Our best transduction level for RRV/FIV (dose of 1.0 × 108 TU) was 26 β-galactosidase-positive cells/20× field (6% of total liver cells), whereas our best transduction level for VSV-G/FIV (dose of 6.8 × 108 TU) was 15 positive cells/20× field (3.5% of the total liver cells).
Both the RRV- and VSV-G-pseudotyped vectors transduced the liver in a dose-dependent manner. However, as shown in Fig. 2D and E, RRV/FIV transduced the liver better than VSV-G/FIV, with an approximately 20-fold-higher efficiency. For example, the mice receiving 3 × 107 TU of RRV/FIV vector had a mean β-galactosidase expression level of 8.4 ± 2.4 cells per ×20 field. It required injection of at least 65 × 107 TU of VSV-G/FIV vector in order to achieve similar expression levels. Another way of analyzing the data is to consider the amount of liver cell transduction in relation to the multiplicity of infection (MOI). If we assume the majority of the particles injected went to the liver and we estimate the mouse liver to have 108 cells and then use an MOI of 0.3 (0.3 TU/cell), RRV/FIV transduced an average of 2% of the liver cells. In contrast, at a much higher MOI (i.e., 3.5), VSV-G/FIV transduced only 0.5% of the liver cells.
Of important note, intravenous injection of RRV/FIV vector did not cause an elevation of SGOT and SGPT levels in the recipient mice, in contrast to intravenous delivery of VSV-G/FIV (Fig. 2F). These elevations of SGOT and SGPT are indicative of tissue damage, as seen with myocardial infarction, liver necrosis, or hepatitis. Since similar amounts of p24 were injected (p24, 42.2 ± 18 μg/ml for VSV-G/FIV; p24, 42.7 ± 13.9 μg/ml for RRV/FIV), the rise in SGOT and SGPT was most likely an effect of the VSV-G rather than the particle load. The results suggest that the RRV Env glycoproteins may have a better safety profile than VSV-G when used intravenously. However, because our preparations were cell culture supernatant concentrates and not purified vector particles, additional studies are required.
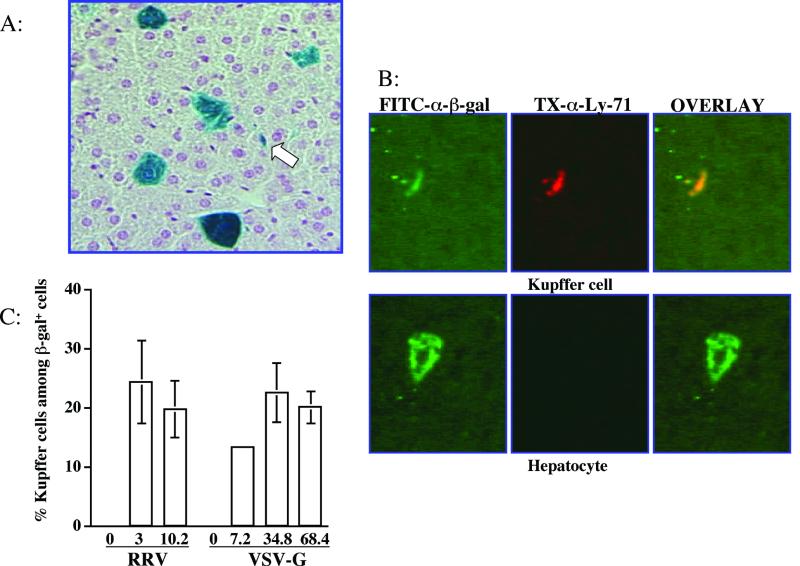
We also characterized the cell types expressing β-galactosidase in the liver. Hepatocytes were the most abundant cell type transduced. However, a significant proportion of the X-Gal-positive cells in the livers from mice receiving either the VSV-G- or RRV-pseudotyped vector morphologically resembled Kupffer cells (Fig. 3A). These β-galactosidase-positive, nonhepatocyte cells lined or were located in the liver sinusoidal spaces and had sparse cytoplasm and a single nucleus, characteristic of Kupffer cells. Transduced liver cells of other types, such as endothelial cells, were not observed. Immunostaining was performed to assess colocalization of β-galactosidase with a Kupffer cell-specific marker, Ly-71. As shown in Fig. 3B, β-galactosidase and Ly-71 doubly positive cells were readily apparent. The percentages of Kupffer cells among the transduced cells were not significantly different between RRV and VSV-G glycoproteins (Fig. 3C). Moreover, the frequency of Kupffer cell transduction with the RRV- or VSV-G pseudotyped vectors was dose independent (Fig. 3C).
FIG. 3.
RRV/FIV vector transduces both hepatocytes and Kupffer cells. (A) The X-Gal-stained liver harvested 3 weeks after injection of 3 × 107 TU of RRV/FIV vector was sectioned and counterstained with nuclear fast red. Blue cells represent the β-galactosidase-expressing cells. The arrow indicates cells morphologically resembling Kupffer cells. (B) Immunostaining was performed to colocalize β-galactosidase expression and the Kupffer cell specific marker, Ly-71 (F4/80). Cells of colocalized β-galactosidase expression (green) and Kupffer cell-specific marker (red) appear yellow in the third merged images. (C) Summary of the percentages of Kupffer cells among the transduced cells in mice receiving various dosages of either RRV/FIV vector or VSV-G/FIV vector. The numbers in the x axis represent the vector doses injected per mouse (107 TU).
We observed the production of antibody responses against β-galactosidase in ∼50% of all of the recipient mice (Fig. 4A). The levels of antibody production were comparable in the groups of mice receiving either the RRV pseudotype or the VSV-G pseudotype (Fig. 4B). The generation of anti-β-galactosidase antibodies appeared unrelated to the number of injections, since antibody production was observed in mice receiving either a single bolus of injection or two consecutive injections. Furthermore, the relative extent of Kupffer cell transduction did not appear to be related to the generation of anti-β-galactosidase antibodies (Fig. 4A). The antibody production against viral p24 protein was minimal in all animals in both RRV and VSV-G groups (<1/800).
FIG. 4.
Antibody immune responses against β-galactosidase and the viral protein (p24) were comparable in mice receiving RRV/FIV vector or VSV-G/FIV vector and unrelated to the extent of Kupffer cell transduction. (A) Summary of the relationship between the percentages of transduced Kupffer cells and the production of antibody immune responses against β-galactosidase. The y axis represents the percentages of transduced Kupffer cells. The left-hand x axis represents mice that did not have antibody immune responses against β-galactosidase. The right-hand x axis indicates mice that developed antibody immune responses against β-galactosidase. Each symbol represents an individual animal. (B) Mice were intravenously injected via the tail vein with a single bolus of control buffer, 10 × 107 TU of RRV/FIV vector, or 35 × 107 TU of VSV-G/FIV vector. Serum samples were collected 3 weeks postinjection. β-Galactosidase-specific (left panel) and p24-specific (right panel) IgG and IgM antibody production were measured as described in Materials and Methods. Note that the scale of serum dilutions for β-galactosidase-specific antibodies is 2 logs higher than that for p24 specific antibodies.
Pseudotyping FIV vector with the RRV envelope glycoproteins directs transgene expression in neuroglia.
Due to the brain-blood barrier, FIV vectors administered intravenously rarely reach the central nervous system in sufficient quantity to cause significant gene expression. Central nervous system tissue consists of two cell types: neurons and neuroglia. Neuroglial cells include astrocytes, oligodendrocytes, and microglia. Whereas neurons are involved in information transmisson, neuroglia support and protect neurons and participate in neural activity, neural nutrition, and the defense processes of the central nervous system.
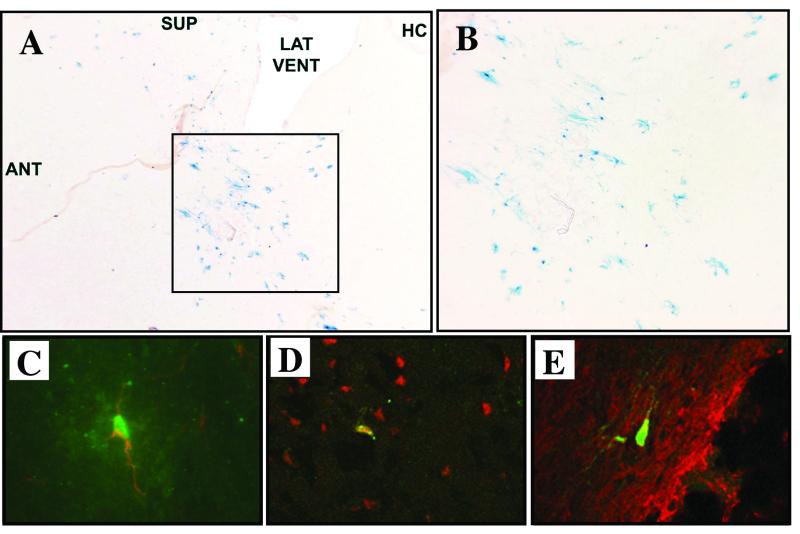
Previously, by using direct injection into brain, we and others demonstrated that lentiviral vectors pseudotyped with VSV-G mainly transduced neurons (1, 2, 4, 6, 17, 26). Wild-type RRV, although predominantly associated with arthritis, has been shown to cause encephalitis (46), suggesting that the RRV envelope glycoproteins may similarly direct neuronal tropism. To examine this possibility, 12 adult C57BL/6 mice underwent striatal injections of 5 × 105 TU of RRV/FIV. At 3 weeks postinjection, X-Gal staining of parasagittal sections revealed β-galactosidase-positive cells distributed in the striatum (Fig. 5A and B), in the corticospinal tract, beneath the corpus callosum, and in the proximal rostral migratory stream (not shown). We did not observe any inflammation at the injection site. This is similar to reports of little inflammation after brain injection with VSV-G-pseudotyped lentiviruses (1, 2, 4, 6, 17, 26, 31). To characterize the cell types transduced, dual immunofluorescent staining was performed for β-galactosidase and NeuN (neurons), β-galactosidase and GFAP (astrocytes), and β-galactosidase and CNPase (oligodendrocytes). While transgene-expressing cells of all three types were detected (Fig. 5C-E), only 7.0% ± 6.5% of β-galactosidase-positive cells were neurons, whereas 56.5% ± 17.2% were astrocytes and 26.6% were oligodendrocytes. This is in striking contrast to the neuronal tropism observed with VSV-G-pseudotyped FIV or HIV vectors (6, 4, 31) where, after striatum injection, the majority of the transduced cells were neurons (89% NeuN positive). These results indicate that pseudotyping with the RRV Env glycoproteins imparts glial cell tropism to FIV.
FIG. 5.
RRV-pseudotyped FIV vector directs transgene expression predominantly in neuroglia. (A) At 3 weeks postinjection of 5 × 105 TU of RRV/FIV vector into the striatum of adult mice, X-Gal staining of 10-μm parasagittal brain sections shows the presence of β-galactosidase-expressing cells (ANT, anterior; SUP, superior; LAT VENT, lateral ventricle; HC, hippocampus). (B) Enlargement of the transduced area indicated in panel A. (C to E) Confocal analysis of brain sections after dual immunofluorecent staining for β-galactosidase (green) and cell-specific markers (red) indicates that astrocytes (C), neurons (D), and oligodendrocytes (E) were transduced. Merged red and green images are shown, and colocalized areas appear yellow.
Pseudotyping FIV vector with the RRV envelope glycoproteins directs minimal transgene expression in skeletal muscle.
VSV-G/FIV effectively transduces hamster skeletal muscle after direct injection (24). However, we found that VSV-G/FIV vector transduces murine muscle very poorly in comparison to the hamster (C. S. Stein et al., unpublished results). Here we tested whether the RRV glycoproteins might allow for enhanced transduction of murine skeletal muscle. Nine adult mice received bilateral quadriceps injections with 9 × 105 TU of RRV/FIV encoding β-galactosidase. A separate group of four mice similarly received 2.7 × 107 TU of VSV-G-pseudotyped vector. At 3 weeks postinjection, X-Gal staining of cross sections of the left quadriceps indicated that five of seven mice were positive for transgene-expressing myocytes after RRV-pseudotyped vector injection. The total number of transduced myocytes varied between animals and ranged from 1 to 15. This level of transduction was similar to that observed after injection of VSV-G-pseudotyped vector, where four of the four animals were positive, with from 1 to 12 transgene-expressing myocytes detected. Representative images from positive mice are shown in Fig. 6A (RRV) and B (VSV-G). Quantification of enzyme activity in lysates of the right quadriceps (Fig. 6B) corresponded with the histochemical analysis. Six of the nine mice that had received RRV/FIV vector exhibited significant β-galactosidase activity (≥2-fold greater than the background level), ranging from 2.5- to 5.6-fold over background, whereas three of the four mice that had received VSV-G/FIV vector exhibited significant β-galactosidase levels (2.3-, 3.5-, and 6.4-fold greater than the background level). Inasmuch as a 30-fold-lower TU of RRV/FIV was introduced into the muscle, these results suggest that RRV-pseudotyped FIV vector transduces murine skeletal muscle more efficiently than VSV-G-pseudotyped FIV vector, albeit poorly relative to other vector systems.
FIG. 6.
Transduction of skeletal muscle. RRV or VSV-G/FIV vectors (30-μl portions) were bilaterally injected into the quadriceps of adult mice. Few β-galactosidase-expressing myocytes were detected in X-Gal-stained cross sections of the left quadriceps, 3 weeks after injection with either RRV (A)- or VSV-G (B)-pseudotyped FIV. (C) Quantification of β-galactosidase activity in the right quadriceps indicated that six of nine mice in the RRV group and three of four mice in the VSV-G group had β-galactosidase levels of >2-fold background (each bar represents an individual animal).
RRV-pseudotyped FIV vectors transduce airway epithelial cells inefficiently.
The potential of using FIV vector pseudotyped with the RRV envelope glycoproteins for gene transfer to the airways was examined with in vitro and in vivo models. In vitro gene delivery of RRV-pseudotyped FIV vector was tested on the following airway epithelial cell lines: IB3 (56), HBE (11), H441 (ATCC HTB-174), A549 (ATCC CCL-185), and primary cultures of well-differentiated human airway epithelia (51, 55). A549 and H441 cell lines are derived from human lung carcinomas, and IB3 and HBE cell lines are transformed human airway cells. Gene transfer efficiency was assessed by applying the viral vector to the cells at limiting dilutions, followed by X-Gal staining after 4 days, as previously described (51). As shown in Table 2, the RRV-pseudotyped vector efficiently transduced the fibroblast-derived HT1080 cells; however, when applied to the airway cell lines, only low-level transduction was detected. In addition, when RRV/FIV was applied to either the apical or the basolateral surface of primary cultures of well-differentiated human airway epithelia, little transduction was observed (data not shown).
TABLE 2.
RRV/FIV transduction of airway epithelial cell linesa
| Cell line | Maximum titer | n |
|---|---|---|
| HT1080 | 3.6 × 108 | >6 |
| A549 | 2 × 103 | 2 |
| HBE | 1 × 102 | 2 |
| H441 | 3.2 × 103 | 2 |
| IB3 | <102 | 2 |
Limiting dilutions of RRV/FIV were applied to HT1080 cells and airway epithelial cell lines, and cells were X-Gal stained 4 days later.
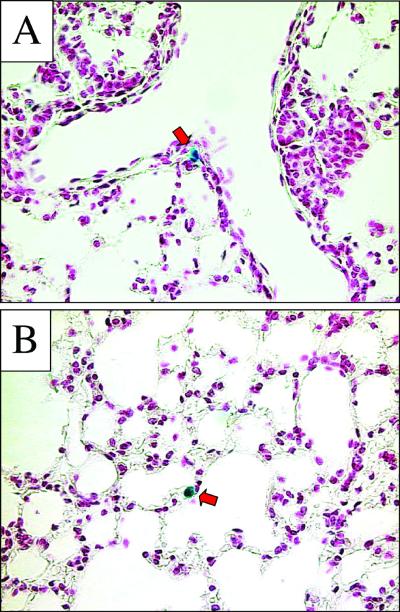
To test the hypothesis that RRV/FIV may transduce the airways via cell types not represented by the in vitro models selected, we delivered the vector to murine respiratory cells in vivo. Five mouse lungs were examined 2 weeks after the instillation of ∼5 × 107 TU of RRV/FIV (delivered over two consecutive days). Microscopic analysis of sectioned lung tissue revealed occasional β-galactosidase-positive cells along the conducting airways (Fig. 7A), as well as alveolar epithelial cells (Fig. 7B); however, the overall gene transfer efficiency was low. As a positive control, we intranasally instilled β-galactosidase-expressing recombinant adenovirus (serotype 5) formulated with 6 mM EDTA. The use of EDTA is necessary to achieve transduction with recombinant adenovirus applied to the apical surface of airway epithelia (51, 52, 55). EDTA serves to disrupt epithelia tight junctions, allowing the adenovirus access to the basolaterally localized CAR receptors (51, 52, 55). This resulted in abundant β-galactosidase expression (data not shown), thus verifying the efficacy of the intranasal delivery protocol. These data suggest that the RRV/FIV is unable to efficiently transduce airway epithelial cells from the apical side.
FIG. 7.
RRV glycoprotein-pseudotyped FIV vector transduces airway cells inefficiently following pulmonary delivery. Five mice were examined 2 weeks after nasal instillation of RRV/FIV vector. As indicated by red arrows, occasional cells along the conducting airways (A) and alveolar epithelium (B) were found to be β-Gal positive. Tissue sections were counterstained with nuclear fast red.
DISCUSSION
Important requirements for successful gene therapy include efficient and specific delivery of the transgene into target cells, stable expression of the transgene, and minimal side effects for the recipients. In the present study, we examined in vivo transgene expression after gene transfer with a nonprimate FIV lentiviral vector pseudotyped with an alphavirus, RRV, envelope glycoproteins. Our data indicate that the RRV glycoproteins can be used to pseudotype FIV lentiviral vector. Moreover, FIV vectors pseudotyped with the RRV glycoproteins have an improved hepatocyte transduction efficiency and lower cytotoxicity than those pseudotyped with VSV-G, one of the most commonly used viral glycoproteins. Furthermore, after injection into the brain, RRV-pseudotyped FIV vectors directed transgene expression predominantly in glial cells, exhibiting a cellular tropism in the central nervous system that is distinct from the VSV-G pseudotype.
The cellular receptors responsible for the binding of the RRV envelope glycoproteins are currently unknown. RRV, as well as its alphavirus relatives Semliki Forest and Sindbis viruses, have extremely broad host ranges. They infect both invertebrate and vertebrate animals and a wide variety of cell types (41, 46). This feature suggests that alphaviruses might utilize multiple receptors to gain access to the cells or tissues or, alternatively, that these viruses use ubiquitous proteins as cellular receptors that are well conserved among diverse species (22, 45). Moreover, even though RRV, Semliki Forest virus, and Sindbis virus all belong to the alphavirus family, they appear to utilize different cellular molecules as receptors for cell binding and/or entry. For example, the glycosaminoglycan heparan sulfate is shown to participate in the binding of Sindbis virus to cells (8). Enzymatic removal of heparan sulfate or the use of heparan sulfate-deficient cells leads to a large reduction in Sindbis virus binding. However, these treatments have no effects on RRV binding, suggesting that, unlike Sindbis virus, RRV virus does not utilize heparan sulfate as a cellular receptor (8). This difference in the use of cellular receptors may explain in part the discrepancy in the cellular tropism in the central nervous system between RRV-pseudotyped FIV vector and Sindbis virus/Semliki Forest virus-derived recombinant vectors (12, 16, 21, 49). Gwag et al. demonstrated that recombinant replication-defective Sindbis virus selectively infected neurons with little transduction of glial cells (21). In contrast, our results suggest that the FIV pseudotyped with RRV envelope glycoproteins preferentially transduces neuroglia.
Compared to the VSV-G protein, the RRV glycoproteins may offer several advantages for pseudotyping FIV-based lentiviral vectors. RRV/FIV transduces hepatocytes more efficiently than VSV-G. Importantly, there was no increase in liver enzymes suggestive of hepatotoxicity at 24 h after systemic administration of RRV/FIV. This is in contrast to results obtained by Park et al. showing elevated SGOT and SGPT levels after administration of a VSV-G-pseudotyped HIV lentiviral vector (34, 35). Additionally, Sharkey et al. previously reported the generation of a stable packaging cell line for the production of RRV-pseudotyped retroviruses (41). No toxic effects were observed in packaging cells stably expressing the RRV glycoproteins (41). The ability to generate stable packaging cell lines is important since this would allow for large-scale production of vectors for potential clinical applications.
RRV viruses are arthropod-borne (transmitted by bites) and therefore must be stable in the bloodstream (46). The RRV viral glycoproteins consist of two transmembrane proteins, E1 and E2, which associate to form a trimeric spike. These spikes produce a well-defined icosahedral structure with T=4 symmetry (54). This icosahedral structure is very different from retroviruses and may be responsible for the stability of RRV-pseudotyped vectors during the centrifugation and storage. Importantly, E2 and E1 are involved in separate processes of virus binding and fusion, respectively. Thus, modification of the E2 subunit may not interfere with the virus fusion process. Indeed, modification of the Sindbis virus E2 subunit glycoprotein by insertion of the staphylococcal protein A sequence has been reported and shows promise in targeting antibody-coated virus to specific cell surface receptors (30, 32).
Our data demonstrate that up to 6% of liver cells were transduced after a single intravenous administration of conventionally prepared RRV/FIV. Although this may be suboptimal for some uses, it should be noted that the recipients receive no pretreatments such as growth factors, toxic injury, or hepatectomy to induce cell proliferation. It is possible that cell proliferation induced by growth factors or surgical procedures can further increase the transduction efficiency with lentiviral vectors (5, 34). It is also very likely that the transduction efficiency can be further enhanced by other delivery methods designed to increase vector MOI for hepatocytes, including catheter directed delivery to the hepatic artery or vein or direct portal vein injection of the vector solution. The enhanced hepatocyte transduction efficiency with the RRV pseudotype likely reflects a greater abundance or accessibility of cellular receptors for RRV. It remains possible, however, that the HT1080 titering cells may have less cellular receptors for RRV glycoproteins than do hepatocytes. Thus, we may underestimate the titers of RRV pseudotyped vector preparations. While the p24 levels have been used to estimate the delivered dose of lentiviral vector preparations, this assay fails to distinguish infectious virions from noninfectious, dead particles. So far there is no single assay that accurately reflects true viral vector titers. Nevertheless, RRV/FIV undeniably transduces a significant proportion of hepatocytes. This finding is very encouraging and indicates its potential as a gene transfer vector.
Antibody-mediated immune responses against the transgene product were observed in approximately half of the mice receiving either the RRV- or VSV-G-pseudotyped FIV vector. The underlying mechanisms for the production of immune responses in some of the recipient mice but not in others are not known. The generation of antibodies against the transgene product was unrelated to the viral glycoproteins used, the number of injections, or the frequency of Kupffer cell transduction. With vectors such as the first generation adenoviral vectors that cause a large inflammatory response in the liver, Kupffer cells may have antigen-presenting functions. Hence, these tissue macrophages present an obstacle for adenovirus vector-mediated gene transfer to internal organs (53). As reported by Wolff et al., depletion of Kupffer cells resulted in an absolute increase in transgene expression, and a delayed clearance of adenovirus vector DNA and transgene expression (53). However, unlike adenoviral vectors, lentiviral vectors cause little inflammation (25, 31). Under noninflammatory conditions, Kupffer cells express little major histocompatibility complex class II and are not thought to be normally involved in the production of immune responses (23). The generation of immune responses in the mice in our study may relate to the extent of transduction of antigen-presenting cells present in other tissues such as the spleen, which was not examined.
We also evaluated the in vivo gene transfer efficiency of the RRV-pseudotyped FIV vector in skeletal muscle and airway epithelial cells. Although some gene transfer was observed in both tissues, the levels of expression were low. These results suggest that the receptor abundance for the RRV glycoprotein is low in these tissues. Alternatively, the receptors may be poorly accessible or there may be inhibition of transduction at steps postbinding (15, 20). Several other envelopes from viruses that have specific tropism for the respiratory tract, including jaagsiekte sheep retrovirus (38) and filoviruses (9), are currently being tested for efficacy in airway epithelial cells.
The present study demonstrates that FIV particles incorporating the RRV envelope glycoproteins can be successfully produced at high titers sufficient for in vivo gene transfer. The RRV-pseudotyped FIV vector shows enhanced hepatocyte gene transfer, improved safety profile, and distinct cellular tropism in the central nervous system. These results suggest potential applications for using the RRV-pseudotyped FIV vector for the correction of hepatocyte-related or neurodegenerative diseases.
Acknowledgments
Y.K. and C.S.S. contributed equally to this study.
We acknowledge the assistance of Melissa Hickey, Diane Tran, Guoshun Wang, and Jeffrey Kasperski and the support of the Vector Core, Cell Culture Core, and Cell Morphology Core. These cores were partially supported by the Cystic Fibrosis Foundation, NHLBI (PPG HL-51670), and the Center for Gene Therapy for Cystic Fibrosis (NIH P30 DK-54759).
This work was supported by the NIH (grants DK53438, HD33531, NS34568, HL61460, HL67623, and P30 DK54759), the Roy J. Carver Trust (B.L.D.), and the National Hemophilia Foundation (Y.K.). We also acknowledge the support of the Indiana Elks Charities Inc. (D.A.S.) and a pilot and feasibility grant from the Center for Gene Therapy for Cystic Fibrosis (D.A.S.).
REFERENCES
- 1.Alisky, J. M., S. M. Hughes, S. L. Sauter, D. Jolly, T. W. Dubensky, Jr., P. D. Staber, J. A. Chiorini, and B. L. Davidson. 2000. Transduction of murine cerebellar neurons with recombinant FIV and AAV5 vectors. Neuroreport 11:2669-2673. [DOI] [PubMed] [Google Scholar]
- 2.Bensadoun, J. C., N. Deglon, J. L. Tseng, J. L. Ridet, A. D. Zurn, and P. Aebischer. 2000. Lentiviral vectors as a gene delivery system in the mouse midbrain: cellular and behavioral improvements in a 6-OHDA model of Parkinson's disease using GDNF. Exp. Neurol. 164:15-24. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz, R., H. Ilves, W. Y. Lin, K. Eckert, A. Coward, S. Tamaki, G. Veres, and I. Plavec. 2001. Construction and molecular analysis of gene transfer systems derived from bovine immunodeficiency virus. J. Virol. 75:3371-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomer, U., L. Naldini, T. Kafri, D. Trono, I. M. Verma, and F. H. Gage. 1997. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J. Virol. 71:6641-6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch, A., P. B. McCray, Jr., S. M. Chang, T. R. Ulich, W. S. Simonet, D. J. Jolly, and B. L. Davidson. 1996. Proliferation induced by keratinocyte growth factor enhances in vivo retroviral-mediated gene transfer to mouse hepatocytes. J. Clin. Investig. 98:2683-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks, A. I., C. S. Stein, S. M. Hughes, J. Heth, P. B. McCray, Jr., S. L. Sauter, J. C. Johnston, D. A. Cory-Slechta, H. J. Federoff, and B. L. Davidson. 2002. Functional correction of established central nervous system deficits in an animal model of lysosomal storage disease with feline immunodeficiency virus-based vectors. Proc. Natl. Acad. Sci. USA 99:6216-6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns, J. C., T. Friedmann, W. Driever, M. Burrascano, and J. K. Yee. 1993. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. USA 90:8033-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrnes, A. P., and D. E. Griffin. 1998. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 72:7349-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan, S. Y., R. F. Speck, M. C. Ma, and M. A. Goldsmith. 2000. Distinct mechanisms of entry by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J. Virol. 74:4933-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffin, J. M. 1999. Retroviridae: the viruses and their replication, p. 1767-1880. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 11.Cozens, A. L., M. J. Yezzi, K. Kunzelmann, T. Ohrui, L. Chin, K. Eng, W. E. Finkbeiner, J. H. Widdicombe, and D. C. Gruenert. 1994. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 10:38-47. [DOI] [PubMed] [Google Scholar]
- 12.Datwyler, D. A., H. M. Eppenberger, D. Koller, J. E. Bailey, and J. P. Magyar. 1999. Efficient gene delivery into adult cardiomyocytes by recombinant Sindbis virus. J. Mol. Med. 77:859-864. [DOI] [PubMed] [Google Scholar]
- 13.DePolo, N. J., J. D. Reed, P. L. Sheridan, K. Townsend, S. L. Sauter, D. J. Jolly, and T. W. Dubensky, Jr. 2000. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol. Ther. 2:218-222. [DOI] [PubMed] [Google Scholar]
- 14.Desmaris, N., A. Bosch, C. Salaun, C. Petit, M. C. Prevost, N. Tordo, P. Perrin, O. Schwartz, H. de Rocquigny, and J. M. Heard. 2001. Production and neurotropism of lentivirus vectors pseudotyped with lyssavirus envelope glycoproteins. Mol. Ther. 4:149-156. [DOI] [PubMed] [Google Scholar]
- 15.Dirks, C., and A. D. Miller. 2001. Many nonmammalian cells exhibit postentry blocks to transduction by gammaretroviruses pseudotyped with various viral envelopes, including vesicular stomatitis virus G glycoprotein. J. Virol. 75:6375-6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan, R. R., A. C. Don-Wauchope, S. Tapechum, M. J. Shipston, R. H. Chow, and P. Estibeiro. 1999. High-efficiency Semliki Forest virus-mediated transduction in bovine adrenal chromaffin cells. Biochem. J. 342(Pt. 3):497-501. [PMC free article] [PubMed] [Google Scholar]
- 17.Englund, U., C. Ericson, C. Rosenblad, R. J. Mandel, D. Trono, K. Wictorin, and C. Lundberg. 2000. The use of a recombinant lentiviral vector for ex vivo gene transfer into the rat CNS. Neuroreport 11:3973-3977. [DOI] [PubMed] [Google Scholar]
- 18.Gatlin, J., M. W. Melkus, A. Padgett, P. F. Kelly, and J. V. Garcia. 2001. Engraftment of NOD/SCID mice with human CD34+ cells transduced by concentrated oncoretroviral vector particles pseudotyped with the feline endogenous retrovirus (RD114) envelope protein. J. Virol. 75:9995-9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghodsi, A., C. Stein, T. Derksen, G. Yang, R. D. Anderson, and B. L. Davidson. 1998. Extensive β-glucuronidase activity in murine central nervous system after adenovirus-mediated gene transfer to brain. Hum. Gene Ther. 9:2331-2340. [DOI] [PubMed] [Google Scholar]
- 20.Goff, S. P. 2001. Intracellular trafficking of retroviral genomes during the early phase of infection: viral exploitation of cellular pathways. J. Gene Med. 3:517-528. [DOI] [PubMed] [Google Scholar]
- 21.Gwag, B. J., E. Y. Kim, B. R. Ryu, S. J. Won, H. W. Ko, Y. J. Oh, Y. G. Cho, S. J. Ha, and Y. C. Sung. 1998. A neuron-specific gene transfer by a recombinant defective Sindbis virus. Brain Res. Mol. Brain Res. 63:53-61. [DOI] [PubMed] [Google Scholar]
- 22.Heil, M. L., A. Albee, J. H. Strauss, and R. J. Kuhn. 2001. An amino acid substitution in the coding region of the E2 glycoprotein adapts Ross River virus to utilize heparan sulfate as an attachment moiety. J. Virol. 75:6303-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janeway, C. A., Jr. 1997. T-cell mediated immunity, p. 7-12. In C. A. Janeway, Jr., and P. Travers (ed.), Immunobiology: the immune system in health and disease, 3rd ed. Current Biology, Ltd., Garland Publishing, Inc., New York, N.Y.
- 24.Johnston, J. C., M. Gasmi, L. E. Lim, J. H. Elder, J. K. Yee, D. J. Jolly, K. P. Campbell, B. L. Davidson, and S. L. Sauter. 1999. Minimum requirements for efficient transduction of dividing and nondividing cells by feline immunodeficiency virus vectors. J. Virol. 73:4991-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kafri, T., U. Blomer, D. A. Peterson, F. H. Gage, and I. M. Verma. 1997. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat. Genet. 17:314-317. [DOI] [PubMed] [Google Scholar]
- 26.Kordower, J. H., M. E. Emborg, J. Bloch, S. Y. Ma, Y. Chu, L. Leventhal, J. McBride, E. Y. Chen, S. Palfi, B. Z. Roitberg, W. D. Brown, J. E. Holden, R. Pyzalski, M. D. Taylor, P. Carvey, Z. Ling, D. Trono, P. Hantraye, N. Deglon, and P. Aebischer. 2000. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science 290:767-773. [DOI] [PubMed] [Google Scholar]
- 27.Mazarakis, N. D., M. Azzouz, J. B. Rohll, F. M. Ellard, F. J. Wilkes, A. L. Olsen, E. E. Carter, R. D. Barber, D. F. Baban, S. M. Kingsman, A. J. Kingsman, K. O'Malley, and K. A. Mitrophanous. 2001. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum. Mol. Genet. 10:2109-2121. [DOI] [PubMed] [Google Scholar]
- 28.Miletic, H., M. Bruns, K. Tsiakas, B. Vogt, R. Rezai, C. Baum, K. Kuhlke, F. L. Cosset, W. Ostertag, H. Lother, and D. von Laer. 1999. Retroviral vectors pseudotyped with lymphocytic choriomeningitis virus. J. Virol. 73:6114-6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, A. D. 1996. Cell surface receptors for retroviruses and implications for gene transfer. Proc. Natl. Acad. Sci. USA 93:11407-11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morizono, K., G. Bristol, Y. M. Xie, S. K. Kung, and I. S. Chen. 2001. Antibody-directed targeting of retroviral vectors via cell surface antigens. J. Virol. 75:8016-8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 32.Ohno, K., K. Sawai, Y. Iijima, B. Levin, and D. Meruelo. 1997. Cell-specific targeting of Sindbis virus vectors displaying IgG-binding domains of protein A. Nat. Biotechnol. 15:763-767. [DOI] [PubMed] [Google Scholar]
- 33.Olsen, J. C. 1998. Gene transfer vectors derived from equine infectious anemia virus. Gene Ther. 5:1481-1487. [DOI] [PubMed] [Google Scholar]
- 34.Park, F., K. Ohashi, W. Chiu, L. Naldini, and M. A. Kay. 2000. Efficient lentiviral transduction of liver requires cell cycling in vivo. Nat. Genet. 24:49-52. [DOI] [PubMed] [Google Scholar]
- 35.Park, F., K. Ohashi, and M. A. Kay. 2000. Therapeutic levels of human factor VIII and IX using HIV-1-based lentiviral vectors in mouse liver. Blood 96:1173-1176. [PubMed] [Google Scholar]
- 36.Peng, K. W., L. Pham, H. Ye, R. Zufferey, D. Trono, F. L. Cosset, and S. J. Russell. 2001. Organ distribution of gene expression after intravenous infusion of targeted and untargeted lentiviral vectors. Gene Ther. 8:1456-1463. [DOI] [PubMed] [Google Scholar]
- 37.Poeschla, E. M., F. Wong-Staal, and D. J. Looney. 1998. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat. Med. 4:354-357. [DOI] [PubMed] [Google Scholar]
- 38.Rai, S. K., J. C. DeMartini, and A. D. Miller. 2000. Retrovirus vectors bearing jaagsiekte sheep retrovirus Env transduce human cells by using a new receptor localized to chromosome 3p21.3. J. Virol. 74:4698-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sajjan, U., G. Thanassoulis, V. Cherapanov, A. Lu, C. Sjolin, B. Steer, Y. J. Wu, O. D. Rotstein, G. Kent, C. McKerlie, J. Forstner, and G. P. Downey. 2001. Enhanced susceptibility to pulmonary infection with Burkholderia cepacia in Cftr−/− mice. Infect. Immun. 69:5138-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnierle, B. S., J. Stitz, V. Bosch, F. Nocken, H. Merget-Millitzer, M. Engelstadter, R. Kurth, B. Groner, and K. Cichutek. 1997. Pseudotyping of murine leukemia virus with the envelope glycoproteins of HIV generates a retroviral vector with specificity of infection for CD4-expressing cells. Proc. Natl. Acad. Sci. USA 94:8640-8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharkey, C. M., C. L. North, R. J. Kuhn, and D. A. Sanders. 2001. Ross River virus glycoprotein-pseudotyped retroviruses and stable cell lines for their production. J. Virol. 75:2653-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein, C. S., and B. L. Davidson. 2002. Gene transfer to the brain using feline immunodeficiency virus-based lentivirus vectors. Methods Enzymol. 346:433-454. [DOI] [PubMed] [Google Scholar]
- 43.Stein, C. S., Y. Kang, S. L. Sauter, K. Townsend, P. Staber, T. A. Derksen, I. Martins, J. Qian, B. L. Davidson, and P. B. McCray, Jr. 2001. In vivo treatment of hemophilia A and mucopolysaccharidosis type VII using nonprimate lentiviral vectors. Mol. Ther. 3:850-856. [DOI] [PubMed] [Google Scholar]
- 44.Stitz, J., C. J. Buchholz, M. Engelstadter, W. Uckert, U. Bloemer, I. Schmitt, and K. Cichutek. 2000. Lentiviral vectors pseudotyped with envelope glycoproteins derived from gibbon ape leukemia virus and murine leukemia virus 10A1. Virology 273:16-20. [DOI] [PubMed] [Google Scholar]
- 45.Strauss, J. H., T. Rümenapf, R. C. Weir, R. J. Kuhn, K. Wang, and E. G. Strauss. 1994. Cellular receptors for alphaviruses, p. 141-163. In E. Wimmer (ed.), Cellular receptors for animal viruses. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 46.Strauss, J. H., and E. G. Strauss. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58:491-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomsen, S., B. Vogt, D. von Laer, C. Heberlein, A. Rein, W. Ostertag, and C. Stocking. 1998. Lack of functional Pit-1 and Pit-2 expression on hematopoietic stem cell lines. Acta Haematol. 99:148-155. [DOI] [PubMed] [Google Scholar]
- 48.Tilton, G. K., T. P. O'Connor, Jr., C. L. Seymour, K. L. Lawrence, N. D. Cohen, P. R. Andersen, and Q. J. Tonelli. 1990. Immunoassay for detection of feline immunodeficiency virus core antigen. J. Clin. Microbiol. 28:898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wahlfors, J. J., S. A. Zullo, S. Loimas, D. M. Nelson, and R. A. Morgan. 2000. Evaluation of recombinant alphaviruses as vectors in gene therapy. Gene Ther. 7:472-480. [DOI] [PubMed] [Google Scholar]
- 50.Wang, G., B. L. Davidson, P. Melchert, V. A. Slepushkin, H. H. G. van Es, M. Bodner, D. J. Jolly, and P. B. McCray, Jr. 1998. Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia. J. Virol. 72:9818-9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, G., V. Slepushkin, J. Zabner, S. Keshavjee, J. C. Johnston, S. L. Sauter, D. J. Jolly, T. W. Dubensky, Jr., B. L. Davidson, and P. B. McCray, Jr. 1999. Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J. Clin. Investig. 104:R55-R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, G, J. Zabner, C. Deering, J. Launspach, J. Shao, M. Bodner, D. J. Jolly, B. L. Davidson, and P. B. McCray, Jr. 2000. Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo. Am. J. Respir. Cell Mol. Biol. 22:129-138. [DOI] [PubMed] [Google Scholar]
- 53.Wolff, G., S. Worgall, N. van Rooijen, W. R. Song, B. G. Harvey, and R. G. Crystal. 1997. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J. Virol. 71:624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao, J., E. G. Strauss, and J. H. Strauss. 1998. Molecular genetic study of the interaction of Sindbis virus E2 with Ross River virus E1 for virus budding. J. Virol. 72:1418-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zabner, J., B. G. Zeiher, E. Friedman, and M. J. Welsh. 1996. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J. Virol. 70:6994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeitlin, P. L., L. Lu, J. Rhim, G. Cutting, G. Stetten, K. A. Kieffer, R. Craig, and W. B. Guggino. 1991. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am. J. Respir. Cell Mol. Biol. 4:313-319. [DOI] [PubMed] [Google Scholar]
- 57.Zhou, W., Y. Zhang, M. S. Hosch, A. Lang, R. M. Zwacka, and J. F. Engelhardt. 2001. Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion. Hepatology 33:902-914. [DOI] [PubMed] [Google Scholar]