Abstract
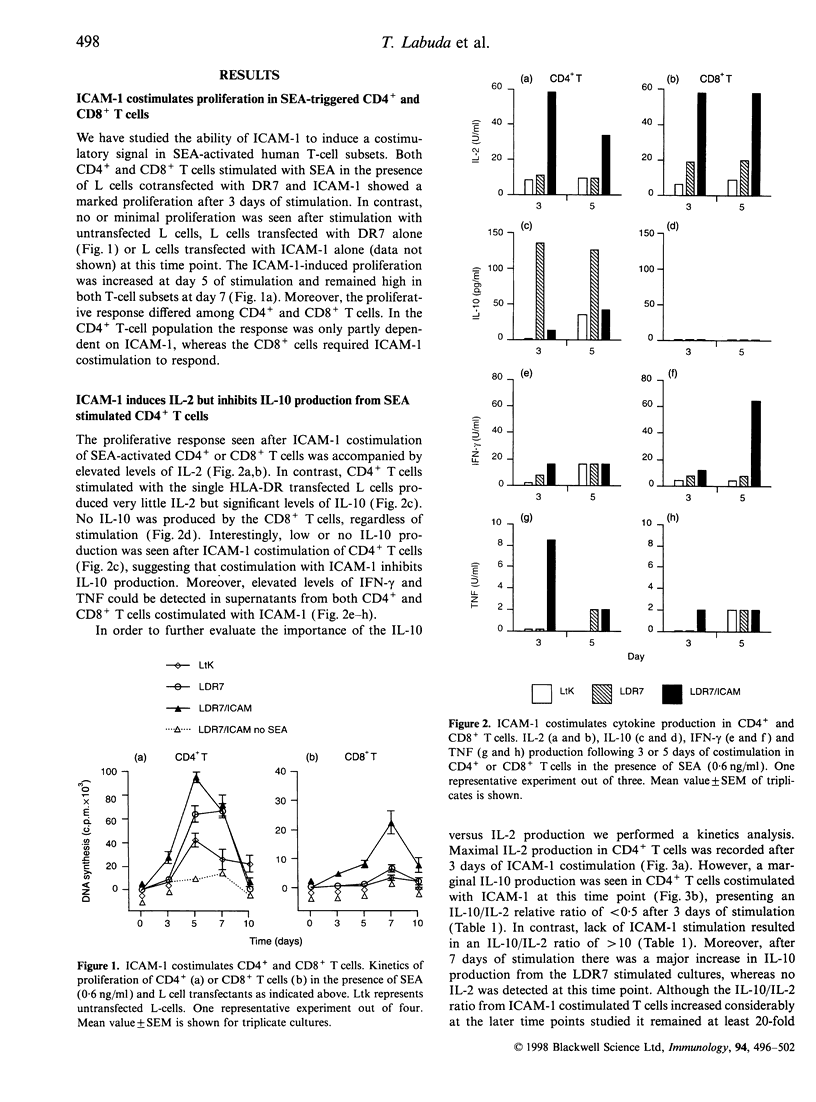
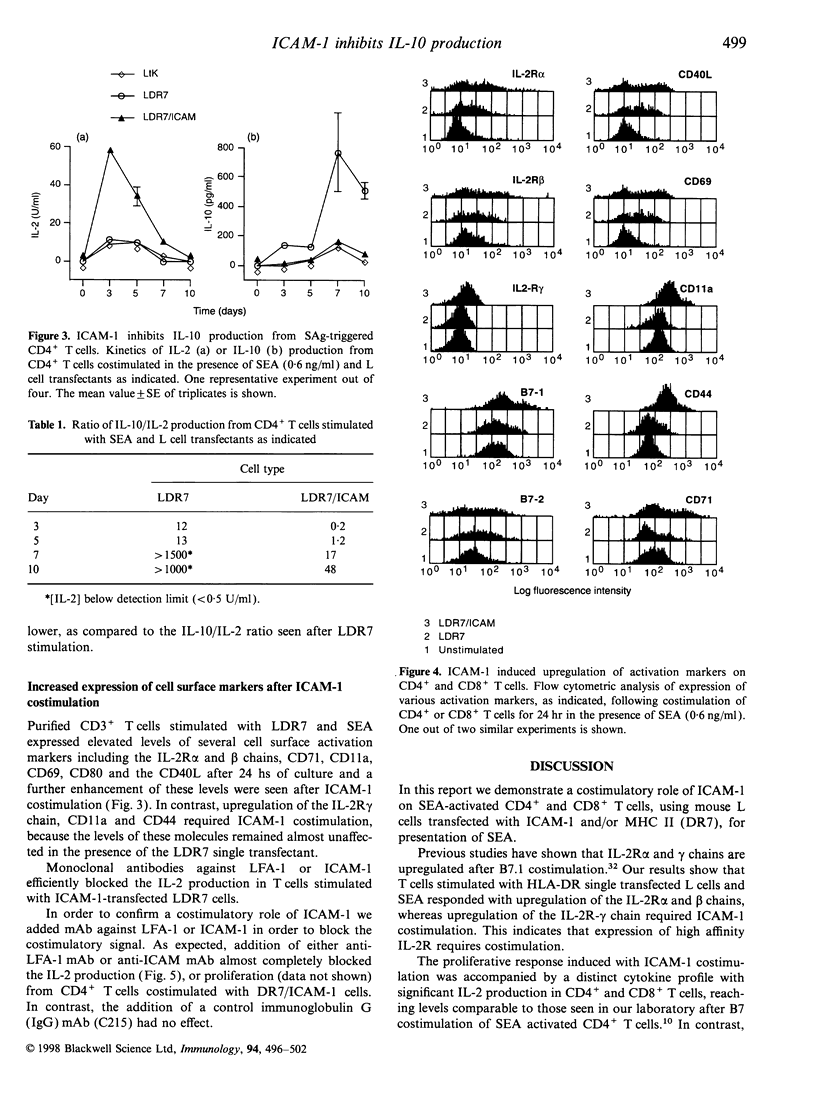
We have previously reported that costimulatory pathways including B7-CD28 and lymphocyte function-associated antigen-3 (LFA-3)-CD2 shape distinct activation profiles in human CD4+ T cells. We now show that superantigen (SAg), in combination with intracellular adhesion molecule-1 (ICAM-1) costimulation drives a proliferative response accompanied by high levels of interleukin-2 (IL-2) and moderate levels of interferon-gamma (IFN-gamma) and tumour necrosis factor (TNF). This response profile differs from that observed in B7 or LFA-3 costimulated T cells because our previous results showed that B7-CD28 costimulation was accompanied by high levels of IL-2, IFN-gamma and TNF, whereas LFA-3 was a potent inducer of IFN-gamma and TNF, but had little influence on IL-2 production. The ICAM-1-induced IL-2 production could efficiently be abrogated with monoclonal antibody (mAb) against ICAM-1 or LFA-1, showing that the activation is dependent of a functional ICAM-1-LFA-1 pathway. SAg-induced IL-2, IFN-gamma and TNF were detected in both CD4+ and CD8+ T cells, whereas production of IL-10 was restricted to CD4+ T cells. A major finding in the present study was that ICAM-1 costimulation strongly inhibits IL-10 production in CD4+ T cells. Our data demonstrate that ICAM-1 costimulation is sufficient to induce large amounts of IL-2. The presence of ICAM-1 results in suppression of IL-10 production in T helper (Th) cells, which may favour the development of Th1 and not Th2 T cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahmsén L., Dohlsten M., Segrén S., Björk P., Jonsson E., Kalland T. Characterization of two distinct MHC class II binding sites in the superantigen staphylococcal enterotoxin A. EMBO J. 1995 Jul 3;14(13):2978–2986. doi: 10.1002/j.1460-2075.1995.tb07300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A., Coggeshall K. M., Mustelin T. Molecular events mediating T cell activation. Adv Immunol. 1990;48:227–360. doi: 10.1016/s0065-2776(08)60756-7. [DOI] [PubMed] [Google Scholar]
- Altmann D. M., Hogg N., Trowsdale J., Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989 Apr 6;338(6215):512–514. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- Azuma M., Cayabyab M., Phillips J. H., Lanier L. L. Requirements for CD28-dependent T cell-mediated cytotoxicity. J Immunol. 1993 Mar 15;150(6):2091–2101. [PubMed] [Google Scholar]
- Boussiotis V. A., Gribben J. G., Freeman G. J., Nadler L. M. Blockade of the CD28 co-stimulatory pathway: a means to induce tolerance. Curr Opin Immunol. 1994 Oct;6(5):797–807. doi: 10.1016/0952-7915(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Wawryk S. O., Burns G. F., Fecondo J. V. Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci U S A. 1988 May;85(9):3095–3099. doi: 10.1073/pnas.85.9.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelens C., Willems F., Delvaux A., Piérard G., Delville J. P., Velu T., Goldman M. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol. 1995 Sep;25(9):2668–2672. doi: 10.1002/eji.1830250940. [DOI] [PubMed] [Google Scholar]
- Bullard D. C., Hurley L. A., Lorenzo I., Sly L. M., Beaudet A. L., Staite N. D. Reduced susceptibility to collagen-induced arthritis in mice deficient in intercellular adhesion molecule-1. J Immunol. 1996 Oct 1;157(7):3153–3158. [PubMed] [Google Scholar]
- Carlsson R., Fischer H., Sjögren H. O. Binding of staphylococcal enterotoxin A to accessory cells is a requirement for its ability to activate human T cells. J Immunol. 1988 Apr 15;140(8):2484–2488. [PubMed] [Google Scholar]
- Carlsson R., Sjögren H. O. Kinetics of IL-2 and interferon-gamma production, expression of IL-2 receptors, and cell proliferation in human mononuclear cells exposed to staphylococcal enterotoxin A. Cell Immunol. 1985 Nov;96(1):175–183. doi: 10.1016/0008-8749(85)90349-1. [DOI] [PubMed] [Google Scholar]
- Cavallo F., Martin-Fontecha A., Bellone M., Heltai S., Gatti E., Tornaghi P., Freschi M., Forni G., Dellabona P., Casorati G. Co-expression of B7-1 and ICAM-1 on tumors is required for rejection and the establishment of a memory response. Eur J Immunol. 1995 May;25(5):1154–1162. doi: 10.1002/eji.1830250504. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Klussman K., Leytze G., Linsley P. S. Proliferation of human T lymphocytes induced with superantigens is not dependent on costimulation by the CD28 counter-receptor B7. J Immunol. 1993 Feb 1;150(3):726–735. [PubMed] [Google Scholar]
- Damle N. K., Klussman K., Linsley P. S., Aruffo A., Ledbetter J. A. Differential regulatory effects of intercellular adhesion molecule-1 on costimulation by the CD28 counter-receptor B7. J Immunol. 1992 Oct 15;149(8):2541–2548. [PubMed] [Google Scholar]
- Davis L. S., Kavanaugh A. F., Nichols L. A., Lipsky P. E. Induction of persistent T cell hyporesponsiveness in vivo by monoclonal antibody to ICAM-1 in patients with rheumatoid arthritis. J Immunol. 1995 Apr 1;154(7):3525–3537. [PubMed] [Google Scholar]
- Dohlsten M., Sjögren H. O., Carlsson R. Histamine inhibits interferon-gamma production via suppression of interleukin 2 synthesis. Cell Immunol. 1986 Sep;101(2):493–501. doi: 10.1016/0008-8749(86)90160-7. [DOI] [PubMed] [Google Scholar]
- Dubey C., Croft M., Swain S. L. Costimulatory requirements of naive CD4+ T cells. ICAM-1 or B7-1 can costimulate naive CD4 T cell activation but both are required for optimum response. J Immunol. 1995 Jul 1;155(1):45–57. [PubMed] [Google Scholar]
- Fawcett J., Holness C. L., Needham L. A., Turley H., Gatter K. C., Mason D. Y., Simmons D. L. Molecular cloning of ICAM-3, a third ligand for LFA-1, constitutively expressed on resting leukocytes. Nature. 1992 Dec 3;360(6403):481–484. doi: 10.1038/360481a0. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Fischer H., Dohlsten M., Andersson U., Hedlund G., Ericsson P., Hansson J., Sjögren H. O. Production of TNF-alpha and TNF-beta by staphylococcal enterotoxin A activated human T cells. J Immunol. 1990 Jun 15;144(12):4663–4669. [PubMed] [Google Scholar]
- Fowell D., Mason D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J Exp Med. 1993 Mar 1;177(3):627–636. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan D. E., Virudachalam S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc Natl Acad Sci U S A. 1997 Jun 10;94(12):6432–6437. doi: 10.1073/pnas.94.12.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987 Feb 1;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh A. F., Davis L. S., Jain R. I., Nichols L. A., Norris S. H., Lipsky P. E. A phase I/II open label study of the safety and efficacy of an anti-ICAM-1 (intercellular adhesion molecule-1; CD54) monoclonal antibody in early rheumatoid arthritis. J Rheumatol. 1996 Aug;23(8):1338–1344. [PubMed] [Google Scholar]
- Kennedy M. K., Picha K. S., Shanebeck K. D., Anderson D. M., Grabstein K. H. Interleukin-12 regulates the proliferation of Th1, but not Th2 or Th0, clones. Eur J Immunol. 1994 Oct;24(10):2271–2278. doi: 10.1002/eji.1830241002. [DOI] [PubMed] [Google Scholar]
- Kennedy M. K., Torrance D. S., Picha K. S., Mohler K. M. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992 Oct 1;149(7):2496–2505. [PubMed] [Google Scholar]
- Koulova L., Clark E. A., Shu G., Dupont B. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med. 1991 Mar 1;173(3):759–762. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Lando P. A., Olsson C., Kalland T., Newton D., Kotb M., Dohlsten M. Regulation of superantigen-induced T cell activation in the absence and the presence of MHC class II. J Immunol. 1996 Oct 1;157(7):2857–2863. [PubMed] [Google Scholar]
- Marcelletti J. F., Ohara J., Katz D. H. Collagen-induced arthritis in mice. Relationship of collagen-specific and total IgE synthesis to disease. J Immunol. 1991 Dec 15;147(12):4185–4191. [PubMed] [Google Scholar]
- Marchant A., Bruyns C., Vandenabeele P., Ducarme M., Gérard C., Delvaux A., De Groote D., Abramowicz D., Velu T., Goldman M. Interleukin-10 controls interferon-gamma and tumor necrosis factor production during experimental endotoxemia. Eur J Immunol. 1994 May;24(5):1167–1171. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Parra E., Wingren A. G., Hedlund G., Björklund M., Sjögren H. O., Kalland T., Sansom D., Dohlsten M. Costimulation of human CD4+ T lymphocytes with B7 and lymphocyte function-associated antigen-3 results in distinct cell activation profiles. J Immunol. 1994 Sep 15;153(6):2479–2487. [PubMed] [Google Scholar]
- Saoudi A., Kuhn J., Huygen K., de Kozak Y., Velu T., Goldman M., Druet P., Bellon B. TH2 activated cells prevent experimental autoimmune uveoretinitis, a TH1-dependent autoimmune disease. Eur J Immunol. 1993 Dec;23(12):3096–3103. doi: 10.1002/eji.1830231208. [DOI] [PubMed] [Google Scholar]
- Schulze-Koops H., Lipsky P. E., Kavanaugh A. F., Davis L. S. Elevated Th1- or Th0-like cytokine mRNA in peripheral circulation of patients with rheumatoid arthritis. Modulation by treatment with anti-ICAM-1 correlates with clinical benefit. J Immunol. 1995 Nov 15;155(10):5029–5037. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Starling G. C., McLellan A. D., Egner W., Sorg R. V., Fawcett J., Simmons D. L., Hart D. N. Intercellular adhesion molecule-3 is the predominant co-stimulatory ligand for leukocyte function antigen-1 on human blood dendritic cells. Eur J Immunol. 1995 Sep;25(9):2528–2532. doi: 10.1002/eji.1830250918. [DOI] [PubMed] [Google Scholar]
- Sundstedt A., Höidén I., Hansson J., Hedlund G., Kalland T., Dohlsten M. Superantigen-induced anergy in cytotoxic CD8+ T cells. J Immunol. 1995 Jun 15;154(12):6306–6313. [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990 Jun 15;144(12):4579–4586. [PubMed] [Google Scholar]
- Vieira P., de Waal-Malefyt R., Dang M. N., Johnson K. E., Kastelein R., Fiorentino D. F., deVries J. E., Roncarolo M. G., Mosmann T. R., Moore K. W. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D., de Vries R. R., Madrigal J. A., Lock C. B., Morgenstern J. P., Trowsdale J., Altmann D. M. Analysis of HLA-DR glycoproteins by DNA-mediated gene transfer. Definition of DR2 beta gene products and antigen presentation to T cell clones from leprosy patients. J Exp Med. 1988 Apr 1;167(4):1442–1458. doi: 10.1084/jem.167.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingren A. G., Dahlenborg K., Björklund M., Hedlund G., Kalland T., Sjögren H. O., Ljungdahl A., Olsson T., Ekre H. P., Sansom D. Monocyte-regulated IFN-gamma production in human T cells involves CD2 signaling. J Immunol. 1993 Aug 1;151(3):1328–1336. [PubMed] [Google Scholar]
- Wu M. X., Ao Z., Hegen M., Morimoto C., Schlossman S. F. Requirement of Fas(CD95), CD45, and CD11a/CD18 in monocyte-dependent apoptosis of human T cells. J Immunol. 1996 Jul 15;157(2):707–713. [PubMed] [Google Scholar]
- Yokota A., Murata N., Saiki O., Shimizu M., Springer T. A., Kishimoto T. High avidity state of leukocyte function-associated antigen-1 on rheumatoid synovial fluid T lymphocytes. J Immunol. 1995 Oct 15;155(8):4118–4124. [PubMed] [Google Scholar]
- de Fougerolles A. R., Stacker S. A., Schwarting R., Springer T. A. Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med. 1991 Jul 1;174(1):253–267. doi: 10.1084/jem.174.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., de Vries J. E. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993 Jun 1;150(11):4754–4765. [PubMed] [Google Scholar]
- van Seventer G. A., Shimizu Y., Shaw S. Roles of multiple accessory molecules in T-cell activation. Curr Opin Immunol. 1991 Jun;3(3):294–303. doi: 10.1016/0952-7915(91)90027-x. [DOI] [PubMed] [Google Scholar]


