Abstract
All coxsackie B (CB) viruses can initiate infection by attaching to the coxsackievirus and adenovirus receptor (CAR). Although some CB isolates also bind to decay-accelerating factor (DAF), the role of DAF interaction during infection remains uncertain. We recently observed that CAR in polarized epithelial cells is concentrated at tight junctions, where it is relatively inaccessible to virus. In the experiments reported here we found that, unlike CAR, DAF was present on the apical surface of polarized cells and that DAF-binding isolates of CB3 and CB5 infected polarized epithelial cells more efficiently than did isolates incapable of attaching to DAF. Virus attachment and subsequent infection of polarized cells by DAF-binding isolates were prevented in the presence of anti-DAF antibody. Serial passage on polarized cell monolayers selected for DAF-binding virus variants. Taken together, these results indicate that interaction with DAF on the apical surface of polarized epithelial cells facilitates infection by a subset of CB virus isolates. The results suggest a possible role for DAF in infection of epithelial cells at mucosal surfaces.
Coxsackie B (CB) viruses, like other enteroviruses, enter the host through the gastrointestinal tract before dissemination to target organs, such as the heart and brain (14). In some cases, coxsackievirus transmission may also occur by way of the respiratory tract. Both the intestine and the airway are lined by polarized epithelial cells whose intercellular tight junctions separate the apical and basolateral surfaces and regulate transepithelial solute flow (13). Coxsackieviruses must either cross or bypass epithelial barriers in the course of infection.
At the cellular level, CB viruses can initiate infection by attaching to the coxsackievirus and adenovirus receptor (CAR), a 46-kDa transmembrane protein that also functions as a receptor for many adenoviruses (3, 19, 23). CAR expression on transfected nonpermissive rodent cells is sufficient to permit infection by all CB viruses that have been tested (12).
In polarized respiratory and intestinal epithelial cells, CAR is absent from the apical surface and is localized to intercellular tight junctions, where it appears to be inaccessible to virus (9). Polarized colonic epithelial cells resist infection by a prototypic strain of coxsackievirus B3 (CB3), CB3-Nancy, unless tight junctions are disrupted and CAR is exposed (9), and sequestration of CAR in tight junctions has impeded efforts to use adenovirus vectors for gene delivery to airway epithelium (15, 24, 25). These observations raise questions about how CB cross mucosal barriers during infection in vivo.
Certain CB viruses interact with an additional cell surface molecule, decay-accelerating factor (DAF, or CD55). Attachment to DAF was first observed with a variant of CB3-Nancy, designated CB3-RD, that had been adapted to growth in rhabdomyosarcoma cells (4, 18). It has also been observed with other isolates of CB1, CB3, and CB5 (21)—as well as with hemagglutinating isolates of other enteroviruses (2, 11, 16, 22, 27)—but not with CB2, CB4, or CB6. Expression of DAF on the surface of transfected rodent cells permits virus attachment but not infection (21, 27), suggesting that DAF, unlike CAR, is incapable of mediating some important postattachment function during virus entry. Although the role of DAF in infection remains uncertain, the DAF-binding capacity demonstrated by a wide variety of enteroviruses suggests that interaction with DAF may serve an important function during infection. We have found that interaction with DAF permits DAF-binding CB isolates to infect polarized epithelial cells, thus surmounting the obstacle presented by CAR sequestration.
MATERIALS AND METHODS
Cell culture.
CHO cells stably expressing CAR (CHO-CAR) and cells transfected with vector alone (CHO-pcDNA) (26) were cultured in nucleoside-free α-minimal essential medium with 10% dialyzed fetal calf serum. To establish polarized monolayers, T84 colonic epithelial cells (provided by Kevin Foskett, University of Pennsylvania) and 16HBE14o− respiratory epithelial cells (provided by Raymond Pickles, University of North Carolina) were cultured in Dulbecco's modified Eagle's medium with 10% fetal calf serum on polyester tissue culture inserts (Costar Transwell Clears; 12-mm diameter, 0.4-μm pore size) until transepithelial resistance, measured with an epithelial voltohmmeter (World Precision Instruments, Sarasota, Fla.), was stable (2,600 to 3,000 Ω · cm2 in 10 to 14 days).
Viruses.
CB3-Nancy and CB3-RD, a variant of CB3-Nancy selected for growth on rhabdomyosarcoma cells, were originally obtained from Richard Crowell (18). This isolate of CB3-Nancy (in contrast to the American Type Culture Collection [ATCC] CB3-Nancy isolate reported by Shafren et al. [21]), does not bind to DAF (4). CB4 strain JVB was obtained from the ATCC. CB5 88-0578, a low-passage clinical isolate provided by John Modlin (Dartmouth Medical School), was previously described (6, 12). Viruses were expanded by growth in HeLa cells and concentrated by ultracentrifugation through a sucrose cushion, and titers were determined by plaque assay using HeLa cells.
For passage of virus on T84 monolayers, a polarized monolayer was exposed to 2 × 107 PFU of CB3-Nancy at room temperature for 1 h. The monolayer was washed and incubated at 37°C for 44 h; no cytopathic changes were evident. Cells and supernatant were frozen and thawed, and virus present in the lysate was expanded on a HeLa cell monolayer, which showed complete cytopathic effect within 24 h. This virus stock was labeled T84P1. Three additional serial passages (T84P2 to T84P4) were performed by exposing fresh T84 monolayers to 0.2 ml of expanded virus stock.
Infection assays.
To determine the susceptibility of cell monolayers to CB infection, we performed immunofluorescence staining for viral antigen. Monolayers were exposed to 10 PFU/cell in a 200-μl volume applied to the apical surface for 1 h at 37°C, washed, and then incubated at 37°C. In preliminary experiments, expression of viral antigen in the human cell lines T84 and 16HBE14o− was clearly detected by 16 h, but in CHO-CAR cells, antigen expression was not detected until 24 to 26 h. To minimize the possibility that virus was undergoing multiple cycles of replication, staining of T84 and 16HBE14o− cells was performed at 16 h, and staining of CHO-CAR cells was performed at 24 to 26 h. When T84 cells were incubated for 24 h before staining, results were qualitatively similar to those observed with cells incubated for 16 h.
Cells were washed in phosphate-buffered saline (PBS), fixed with a 50/50 mixture of methanol and acetone, and stained for CB3 viral antigen using anti-CB3 monoclonal antibody (Chemicon) diluted 1:5 in PBS containing 4% fetal bovine serum. After incubation at 37°C for 30 min, cells were washed with PBS and stained with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse secondary antibody for 30 min at 37°C. The membrane was mounted on slides with mounting medium (Vectashield; Vector Labs). Cells were examined by immunofluorescence microscopy, and digital photographs were taken for quantification of cells expressing viral antigen. In some experiments a commercial blend of anti-CB monoclonal antibodies (Chemicon) was used to detect CB3, CB4, and CB5 in the same experiment. Uninfected cells incubated with anti-CB antibodies and secondary antibody did not reveal positive staining.
Immunofluorescence staining for DAF and CAR.
To examine DAF and CAR localization, polarized T84 monolayers were fixed and permeabilized with 100% methanol, blocked with 10% goat serum, and then stained with affinity-purified rabbit anti-CAR antibody diluted 1:500 in 10% goat serum, followed by Cy3-conjugated goat anti-rabbit secondary antibody. (To produce polyclonal rabbit anti-CAR antibody, rabbits were immunized with the CAR extracellular domain produced in a baculovirus system and antibody was purified by affinity chromatography.) T84 cells were also stained for DAF by using a 1:200 dilution of FITC-conjugated anti-DAF monoclonal antibody IA10 (Pharmingen), and nuclei were detected with 4′,6′-diamidino-2-phenylindole (DAPI). Cells were washed, and the membranes were cut from the tissue culture insert, placed on slides with mounting medium, and examined with a Leica TCS 4D confocal microscope. There was no bleed-through of fluorescence from either the FITC channel into the Cy3 channel or from the Cy3 channel into the FITC channel when cells were stained only for CAR or for DAF.
Inhibition of coxsackievirus infection.
Monoclonal antibodies to DAF (IF7) (2) or CAR (RmcB) (10), or a control monoclonal antibody (MOPC195; Sigma) (ascites fluid diluted 1:50 in medium) were added to polarized T84 cells or CHO-CAR cells and incubated for 1 h at room temperature. In some experiments, rabbit anti-CAR or preimmune serum (9) was used in attempts to block virus interaction with CAR. Virus was then added in the continued presence of these antibodies, and cells were incubated for 1 h at 37°C. Cells were washed, fed with antibody-containing medium, and incubated for 16 h at 37°C before staining for viral antigen. Cells were fixed with 4% paraformaldehyde, washed, incubated with a 1:2,000 dilution of horse anti-CB3 or anti-CB5 serum (ATCC) at room temperature for 1 h, washed, and stained with FITC-conjugated secondary anti-horse antibody for 1 h. Preimmune horse serum did not stain infected cells. Cells were examined with immunofluorescence microscopy.
Radiolabeled virus attachment.
Viruses were labeled by growth on HeLa cells in the presence of [35S]methionine and purified by sucrose gradient centrifugation as described elsewhere (5). To measure virus attachment, T84 cell monolayers in transwell inserts or CHO cells in 24-well plates were incubated with radiolabeled virus (approximately 20,000 cpm in 0.1 ml) for 2 h at room temperature, washed three times with PBS, and then disrupted with Solvable (Packard); bound radioactivity was determined by scintillation counting. In some experiments, antibodies to DAF or CAR were added before virus addition.
RESULTS
CB3-RD infects T84 monolayers.
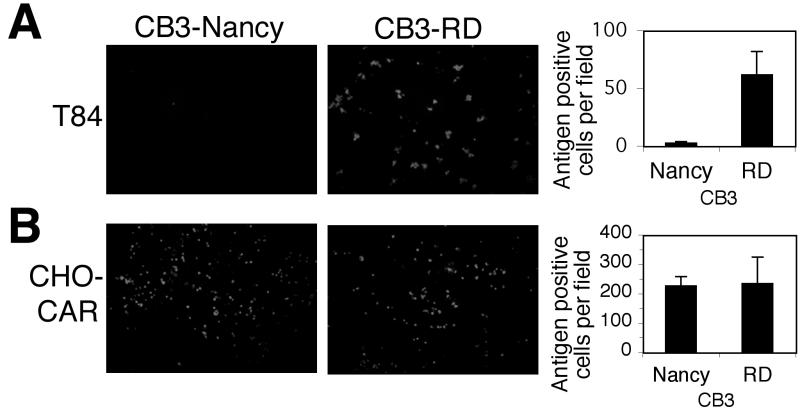
We recently observed that CAR is localized to the tight junction in polarized epithelial cells and that localization of CAR limits infection by CB3-Nancy (9), a virus isolate that binds to CAR (3) but not to DAF (4) on transfected cells. We now examined the susceptibility of polarized epithelial cells to infection by CB3-RD, a virus that, unlike CB3-Nancy, binds to DAF as well as to CAR (4). Polarized monolayers of T84 colonic epithelial cells cultured in transwell filters were exposed to each virus (10 PFU/cell) and stained to detect viral antigen 16 h after infection. CB3-RD infected these cells approximately 30-fold more efficiently than did CB3-Nancy (Fig. 1A). At 60 h after infection, extensive cytopathic changes were observed in monolayers exposed to CB3-RD but not in those exposed to CB3-Nancy (data not shown). Similarly, CB3-RD was more efficient than CB3-Nancy in infecting monolayers of another polarized cell line, 16HBE14o− (data not shown). In control experiments, the two viruses infected nonpolarized CHO-CAR cells with equal efficiency (Fig. 1B).
FIG. 1.
CB3-RD infects polarized T84 cells more efficiently than does CB3-Nancy. Polarized T84 cells (A) or CHO cells expressing CAR (B) were exposed to CB3-RD and CB3-Nancy (10 PFU/cell), washed, and then incubated at 37°C. Cells were fixed and stained with an anti-CB3 mouse monoclonal antibody followed by a FITC-conjugated secondary antibody. Cells were visualized by immunofluorescence microscopy, and viral antigen-positive cells were quantified by examining digital photographs of random fields. Graphs show the average number of antigen-positive cells per field and standard deviations (error bars) for three fields. Results are representative of three experiments.
CB3-RD attaches to T84 monolayers.
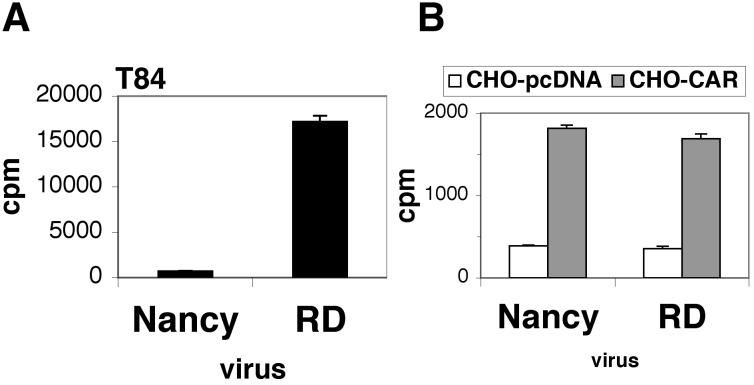
Radiolabeled CB3-RD bound more efficiently to polarized T84 cells than did CB3-Nancy, although both virus strains bound equally well to CHO-CAR cells (Fig. 2). These data suggest that efficient infection of polarized cells by CB3-RD and inefficient infection by CB3-Nancy resulted from the differing capacities of these viruses to attach to the apical cell surface.
FIG. 2.
Attachment of CB3-RD to T84 monolayers is more efficient than attachment of CB3-Nancy. Radiolabeled CB3-RD and CB3-Nancy were added to polarized T84 cells (A) or to nonpolarized CHO-CAR or CHO-pcDNA cells (B). After 2 h of incubation at room temperature, cells were washed and bound virus was determined by scintillation counting. Results are shown as the average cpm of virus bound (± standard deviation) for triplicate monolayers.
DAF is localized to the apical surface of polarized T84 cells.
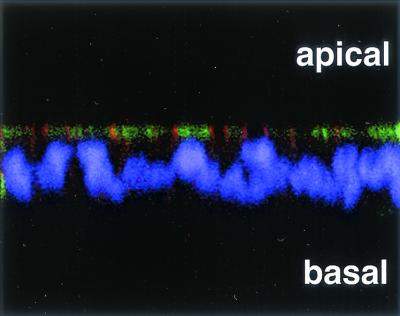
When T84 monolayers were permeabilized, stained with anti-CAR and anti-DAF antibodies, and examined by confocal microscopy, CAR was not detected on the apical surface but, consistent with its localization to tight junctions, was concentrated at the apical pole of the basolateral membrane (Fig. 3, red). In contrast, DAF was abundant on the apical membrane of many cells (Fig. 3, green), consistent with previous observations that the glycolipid membrane anchor of DAF serves as an apical sorting signal in polarized cells (8). DAF staining, but not CAR staining, was seen in unpermeabilized T84 monolayers (data not shown), suggesting that DAF, but not CAR, was accessible on the apical surface. These results indicate that DAF and CAR are expressed at different locations in polarized T84 cells and that DAF is available to interact with virus on the apical cell surface.
FIG. 3.
DAF is expressed at the apical surface of polarized T84 cells. Polarized T84 monolayers were fixed, permeabilized, and stained for DAF by using the anti-DAF antibody IA10 directly conjugated to FITC (green) and for CAR by using an affinity-purified anti-CAR antibody followed by a secondary antibody conjugated to Cy3 (orange-red). Nuclei were stained with DAPI (purple). Stained cells were examined by confocal microscopy; a representative x-z axis cross section of the monolayer is shown.
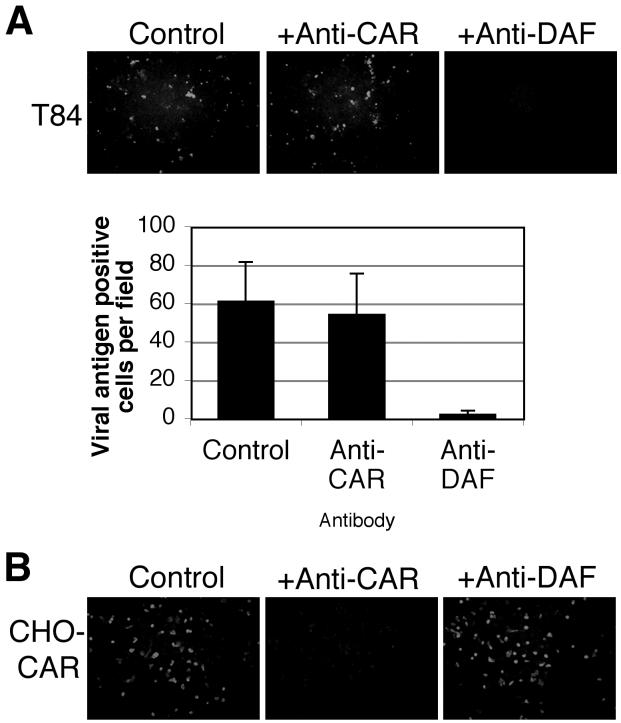
CB3-RD attachment to and infection of T84 monolayers are inhibited by anti-DAF antibody.
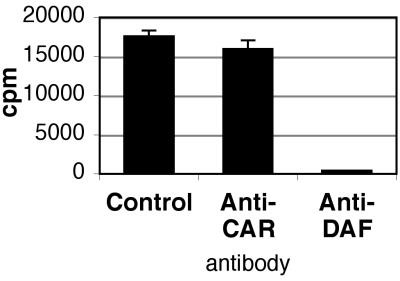
On nonpolarized cells, CB3 interaction with CAR is inhibited by the anti-CAR monoclonal antibody RmcB, and interaction with DAF is inhibited by the anti-DAF monoclonal antibody IF7 (3, 4). On polarized T84 monolayers, virus attachment was dramatically inhibited by IF7 (Fig. 4); in contrast, RmcB—like the control antibody MOPC 195—had little effect on virus attachment. These results suggest a primary role for DAF in mediating CB3-RD attachment to polarized T84 cells. Consistent with this, the anti-DAF antibody, but not the anti-CAR antibody, strongly inhibited CB3-RD infection of T84 monolayers, as measured by viral antigen staining (Fig. 5). Polyclonal rabbit anti-CAR antibody, which blocks virus infection of nonpolarized cells (unpublished data), did not inhibit infection of polarized T84 cells by CB3-RD (data not shown). In control experiments with CHO-CAR cells, RmcB inhibited CB3-RD infection, whereas IF7 did not (Fig. 5). These data suggest that interaction with DAF is important for CB3-RD infection of polarized T84 cells.
FIG. 4.
CB3-RD attachment to polarized T84 cells is inhibited by an anti-DAF antibody. T84 monolayers were preincubated with control monoclonal antibody, anti-CAR monoclonal antibody, or an anti-DAF monoclonal antibody and exposed to radiolabeled CB3-RD at room temperature. Monolayers were washed, and cell-bound radioactivity was determined. A representative experiment is shown; the histogram shows mean cpm bound (± standard deviation) for triplicate samples.
FIG. 5.
Anti-DAF antibody inhibits the infection of polarized T84 cells by CB3-RD. (A) Polarized T84 monolayers were preincubated with control monoclonal antibody, anti-CAR monoclonal antibody, or anti-DAF antibody before infection by CB3-RD. Infected cells were visualized by staining for CB3 viral antigen with a polyclonal horse anti-CB3 serum followed by a secondary antibody conjugated to FITC and examination by immunofluorescence microscopy. The graph shows the average number of viral antigen-positive cells per field (± standard deviation) for three fields. Results are representative of four experiments. (B) Control CHO-CAR cells were preincubated with a control antibody, an anti-CAR antibody, or an anti-DAF antibody before exposure to CB3-RD. Cells were stained for CB3 viral antigen.
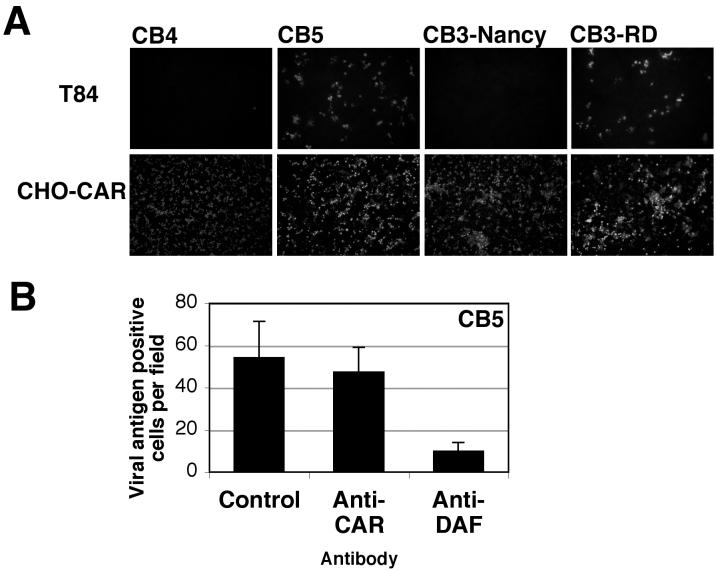
A DAF-binding CB5 clinical isolate also infects T84 monolayers.
We previously found that a CB5 clinical isolate (88-0578; from John Modlin) bound to DAF-transfected CHO cells (20,000 cpm added; 6,696 ± 201 cpm bound to CHO-DAF, 58 ± 4 cpm bound to CHO control), whereas the CB4 strain JVB did not (18,000 cpm added; 134 ± 13 cpm bound to CHO-DAF, 126 ± 1 cpm bound to CHO control, and 1,333 ± 70 cpm bound to CHO-CAR). Both of these virus isolates interact with CAR (12). We tested the capacity of these viruses to infect polarized T84 monolayers. Like CB3-RD, the DAF-binding CB5 isolate infected T84 monolayers efficiently; in contrast, the CB4 isolate did not (Fig. 6A). Infection by the CB5 isolate was markedly inhibited by the anti-DAF antibody (Fig. 6B). These results indicate that binding to DAF facilitates infection of polarized T84 cells by a CB5 isolate as well as by CB3-RD.
FIG. 6.
CB5 infects T84 monolayers, and infection is inhibited by anti-DAF antibody. (A) Polarized T84 cells or CHO-CAR cells were exposed to a CB4 isolate that does not bind DAF, a clinical CB5 isolate that binds DAF, CB3-Nancy, or CB3-RD (10 PFU/cell). After washing, cells were incubated at 37°C. Cells were fixed, stained with a blend of monoclonal antibodies capable of detecting all CB serotypes (Chemicon) followed by a FITC-conjugated secondary antibody, and then examined by immunofluorescence microscopy. Representative fields are shown. (B) Inhibition of CB5 infection of T84 monolayers. Polarized T84 monolayers were preincubated with control monoclonal antibody, anti-CAR monoclonal antibody, or anti-DAF antibody before infection by CB5. Infected cells were visualized by staining for CB5 viral antigen with a polyclonal horse anti-CB5 serum followed by a FITC-conjugated secondary antibody. Viral antigen-positive cells were quantified by examining digital photographs of random fields. The graph shows the mean number of antigen-positive cells per field (± standard deviation) for five fields. Results are representative of three experiments.
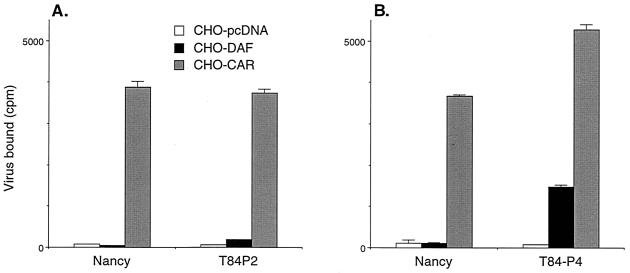
Interaction with T84 monolayers selects for DAF-binding variants.
We performed four serial passages of CB3-Nancy on polarized T84 monolayers. Virus stocks obtained after the second and fourth passages were radiolabeled and their attachment to CHO-DAF cells was measured (Fig. 7). Although CB3-Nancy showed no measurable attachment to CHO-DAF cells, some attachment was noted after two passages of CB3-Nancy on T84 monolayers, and significant attachment was noted after four passages. The results indicate that DAF-binding virus variants enjoy a selective growth advantage in these polarized epithelial cells.
FIG. 7.
Passage of CB3-Nancy on T84 monolayers selects for DAF-binding variants. CB3-Nancy was passaged on T84 monolayers as described in Materials and Methods. After the second (A) and fourth (B) serial passages, viruses were radiolabeled and binding to transfected CHO cell monolayers was measured. The graph shows mean cpm bound (± standard deviation) for triplicate samples.
DISCUSSION
Although all CB viruses can use CAR as a receptor on transfected nonpolarized cells (12), on polarized epithelium CAR is concentrated in tight junctions where it is relatively inaccessible to virus (9). In the experiments reported here, we found that, unlike CAR, DAF was present on the apical surface of polarized cells and that DAF-binding isolates of CB3 and CB5 infected polarized epithelial cells more efficiently than did isolates incapable of attaching to DAF. Virus attachment and subsequent infection of polarized cells by DAF-binding isolates were prevented in the presence of anti-DAF antibody. Serial passage on polarized cell monolayers resulted in enrichment of DAF-binding variants within a virus stock. Taken together, these results indicate that interaction with DAF on the apical cell surface facilitates infection of polarized epithelial cells by a subset of CB isolates.
Virus attachment to CAR on transfected cells leads to infection, whereas attachment to DAF does not (4, 21, 27), suggesting that DAF is by itself incapable of mediating postattachment events essential for virus infection. We suspect that once virus is bound to DAF at the apical surface of an epithelial cell, further interaction with CAR may be necessary for infection to proceed. In these experiments, however, anti-CAR antibodies had little inhibitory effect on infection of polarized epithelial cells by CB3-RD; this suggests that if transfer of DAF-bound virions to CAR occurs, it does so either by a mechanism that is not easily inhibited by antibody or within a cellular compartment that antibody cannot reach. We cannot exclude the possibility that DAF alone is sufficient for infection of these polarized cells. Further work will be required to define the mechanism by which attachment to DAF leads to infection of polarized epithelial cells.
Coxsackieviruses are transmitted by the fecal-oral route, and respiratory transmission may also occur. For poliovirus, both mucosal epithelium (20) and mucosa-associated lymphoid tissue (7) have been proposed to be the primary site of virus replication. Attachment to DAF and replication within epithelial cells of the intestinal tract may augment the capacity of DAF-binding viruses to initiate infection in vivo and thus may play an important part in the pathogenesis of coxsackievirus disease. It is not known whether DAF-binding viruses differ in pathogenicity from those that interact only with CAR. Viruses that do not interact with DAF may avoid the barrier imposed by the tight junction by passing through M cells to reach submucosal lymphoid tissue. It is interesting that reoviruses, which also infect by way of the gastrointestinal tract, interact with junctional adhesion molecule (1), a receptor that—like CAR—is concentrated in tight junctions.
Phylogenetically distinct enteroviruses interact with DAF but differ in their interaction with individual DAF structural domains, suggesting that the capacity to bind DAF may have evolved independently and repeatedly (17). However, the selective advantage afforded to DAF-binding viruses has remained enigmatic. Given that viruses must cross epithelial barriers, DAF-mediated infection of epithelial cells in vivo may account for the widespread occurrence of DAF binding among the human enteroviruses.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL54735 and T32 HL07443-22).
Scott Baldwin and Christopher Cohen provided excellent advice. We thank Richard Crowell and John Modlin for virus isolates. Gary Cohen, Roselyn Eisenberg, and Charles Whitbeck provided generous help in producing the extracellular domain of CAR. We also thank Susan Puhalla for assistance with confocal microscopy, and Jennifer Cunningham, Anita Krithivas, Kate MacNamara, and Tauni Ohman for technical assistance.
REFERENCES
- 1.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson, J. M., M. Chan, K. Solomon, N. F. St. John, H. Lin, and R. W. Finberg. 1994. Decay-accelerating factor, a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc. Natl. Acad. Sci. USA 91:6245-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. G. Mohanty, R. L. Crowell, N. F. St. John, D. M. Lublin, and R. W. Finberg. 1995. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55). J. Virol. 69:1903-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergelson, J. M., N. St. John, S. Kawaguchi, M. Chan, H. Stubdal, J. Modlin, and R. W. Finberg. 1993. Infection by echoviruses 1 and 8 depends on the α2 subunit of human VLA-2. J. Virol. 67:6847-6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berlin, L., M. Rorabaugh, F. Heldrich, K. Roberts, T. Doran, and J. Modlin. 1993. Aseptic meningitis in infants <2 years of age: diagnosis and etiology. J. Infect. Dis. 168:888-892. [DOI] [PubMed] [Google Scholar]
- 7.Bodian, D. 1955. Emerging concept of poliomyelitis infection. Science 122:105-108. [DOI] [PubMed] [Google Scholar]
- 8.Brown, D. A., B. Crise, and J. K. Rose. 1989. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science 245:1499-1501. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, C. J., J. T. C. Shieh, R. J. Pickles, T. Okegawa, J.-T. Hsieh, and J. M. Bergelson. 2001. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 98:15191-15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu, K.-H. L., K. Lonberg-Holm, B. Alstein, and R. L. Crowell. 1988. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J. Virol. 62:1647-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karnauchow, T. M., D. L. Tolson, B. A. Harrison, E. Altman, D. M. Lublin, and K. Dimock. 1996. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J. Virol. 70:5143-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martino, T. A., M. Petric, H. Weingartl, J. M. Bergelson, M. A. Opavsky, C. D. Richardson, J. F. Modlin, R. W. Finberg, K. C. Kain, N. Willis, C. J. Gauntt, and P. P. Liu. 2000. The coxsackie-adenovirus receptor (CAR) is used by reference strains and clinical isolates representing all six serotypes of coxsackievirus group B, and by swine vesicular disease virus. Virology 271:99-108. [DOI] [PubMed] [Google Scholar]
- 13.Mitic, L. L., and J. M. Anderson. 1998. Molecular architecture of tight junctions. Annu. Rev. Physiol. 60:121-142. [DOI] [PubMed] [Google Scholar]
- 14.Modlin, J. F. 1995. Picornaviridae, p. 1606-1613. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed. Churchill Livingstone, New York, N.Y.
- 15.Pickles, R. J., D. McCarty, H. Matsui, P. J. Hart, S. H. Randell, and R. C. Boucher. 1998. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J. Virol. 72:6014-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell, R. M., V. Schmitt, T. Ward, I. Goodfellow, D. J. Evans, and J. W. Almond. 1998. Characterization of echoviruses that bind decay accelerating factor (CD55): evidence that some haemagglutinating strains use more than one cellular receptor. J. Gen. Virol. 79:1707-1713. [DOI] [PubMed] [Google Scholar]
- 17.Powell, R. M., T. Ward, I. Goodfellow, J. W. Almond, and D. J. Evans. 1999. Mapping the binding domains on decay accelerating factor (DAF) for haemagglutinating enteroviruses: implications for the evolution of a DAF-binding phenotype. J. Gen. Virol. 80:3145-3152. [DOI] [PubMed] [Google Scholar]
- 18.Reagan, K. J., B. Goldberg, and R. L. Crowell. 1984. Altered receptor specificity of coxsackie B3 after growth in rhabdomyosarcoma cells. J. Virol. 49:635-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roelvink, P. W., A. Lizonova, J. G. M. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. Brough, E. I. Kovesdi, and T. J. Wickham. 1998. The coxsackie-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabin, A. B. 1956. Pathogenesis of poliomyelitis: reappraisal in the light of new data. Science 123:1151-1157. [DOI] [PubMed] [Google Scholar]
- 21.Shafren, D. R., R. C. Bates, M. V. Agrez, R. L. Herd, G. F. Burns, and R. D. Barry. 1995. Coxsackieviruses B1, B3, and B5 use decay accelerating factor as a receptor for cell attachment. J. Virol. 69:3873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafren, D. R., D. J. Dorahy, R. A. Ingham, G. F. Burns, and R. D. Barry. 1997. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J. Virol. 71:4736-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walters, R. W., T. Grunst, J. M. Bergelson, R. W. Finberg, M. W. Welsh, and J. Zabner. 1999. Basolateral localization of fiber receptors limits adenovirus infection of airway epithelia. J. Biol. Chem. 274:10219-10226. [DOI] [PubMed] [Google Scholar]
- 25.Walters, R. W., W. van't Hof, S. M. Yi, M. K. Schroth, J. Zabner, R. G. Crystal, and M. J. Welsh. 2001. Apical localization of the coxsackie-adenovirus receptor by glycosyl-phosphatidylinositol modification is sufficient for adenovirus-mediated gene transfer through the apical surface of human airway epithelia. J. Virol. 75:7703-7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, X., and J. M. Bergelson. 1999. CAR cytoplasmic and transmembrane domains are not essential for infection by coxsackie B viruses and adenoviruses. J. Virol. 73:2259-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward, T., P. A. Pipkin, N. A. Clarkson, D. M. Stone, P. D. Minor, and J. W. Almond. 1994. Decay accelerating factor (CD55) identified as a receptor for echovirus 7 using CELICS, a rapid immuno-focal cloning method. EMBO J. 13:5070-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]