Abstract
Activated macrophages utilize both reactive oxygen intermediates and reactive oxynitrogen intermediates for defence against microbes. However, simultaneous generation of superoxide (O- 2;) and nitric oxide (NO) could be harmful to host cells due to the production of peroxynitrite, nitrogen dioxide and hydroxyl radicals. Therefore, the regulation of the production of these molecules is critical to host survival. During periods of inflammation or infection, the level of serum C-reactive protein (CRP) increases in many species. Human and rat CRP have been shown to bind and interact with phagocytic cells. Since many of the interactions of CRP involve the binding to the phosphocholine ligand, we studied the role of CRP in O- 2; and NO generation through the modulation of phosphatidylcholine (PC) metabolism in macrophages. This study has shown that, while rat CRP inhibited phorbol myristate acetate- (PMA) induced release of O- 2; by rat macrophages, CRP-treated macrophages released NO in a time- and dose-dependent manner. CRP increased inducible nitric oxide synthase (iNOS) enzyme as well as iNOS mRNA levels in rat macrophages. Tricyclodecan-9-yl-xanthogenate (D609), an inhibitor to PC phospholipase C (PC-PLC), suppressed iNOS induction but enhanced PMA-induced release of O- 2;. These data indicate that an increased level of CRP during periods of inflammation may result in differential regulation of macrophage NADPH oxidase and iNOS activity. Increased hepatic synthesis of CRP may contribute to the mechanism by which phagocytic cells avoid simultaneous O- 2; and NO synthesis, and this could possibly be mediated through the regulation of PC-PLC.
Full text
PDF


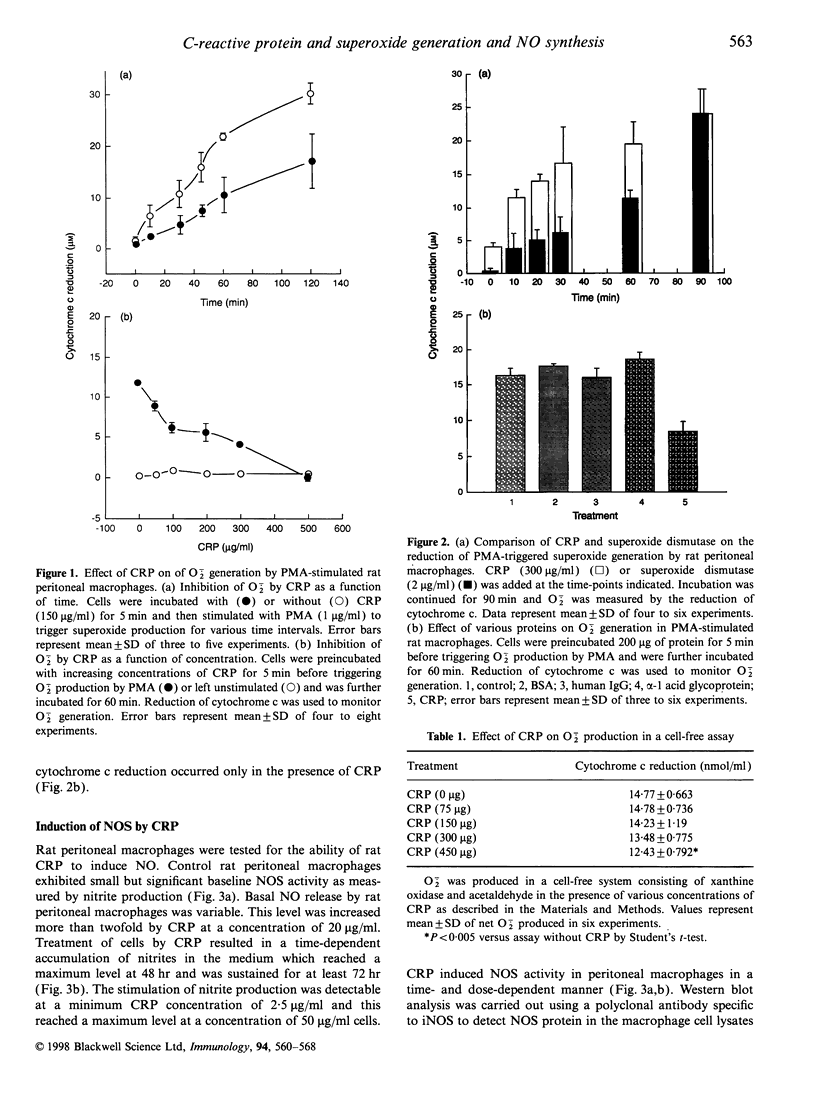
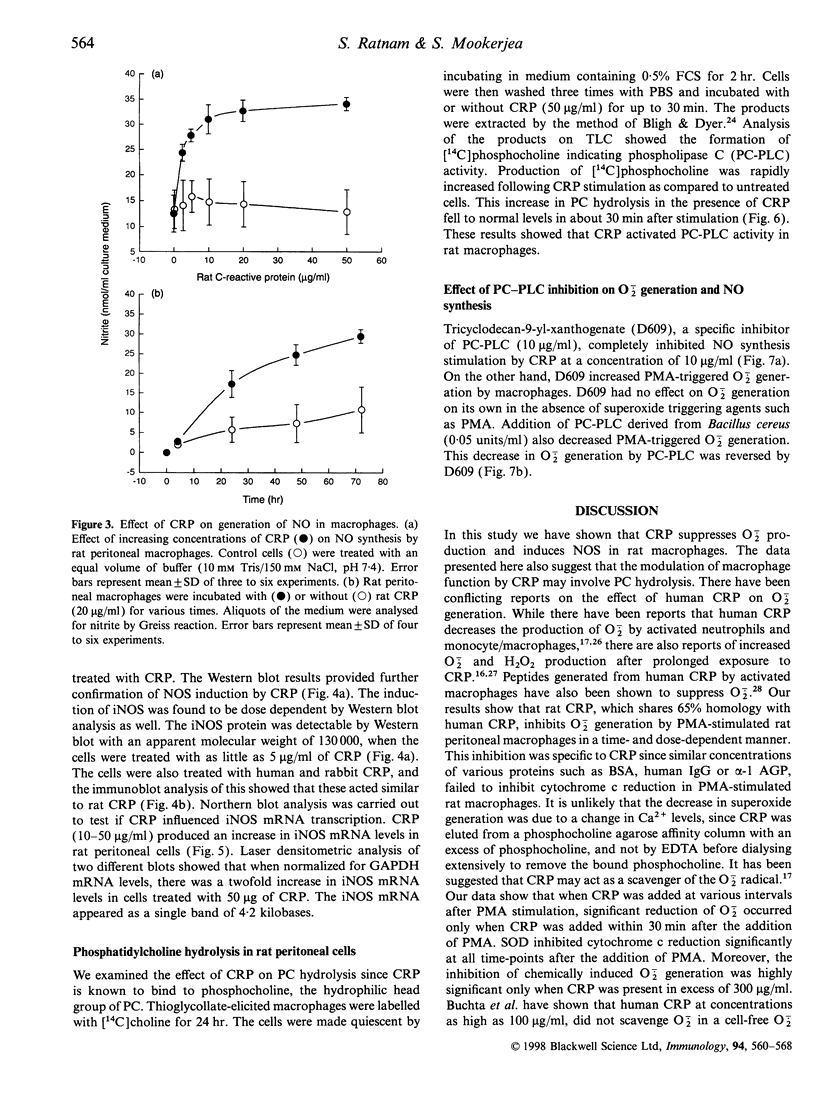
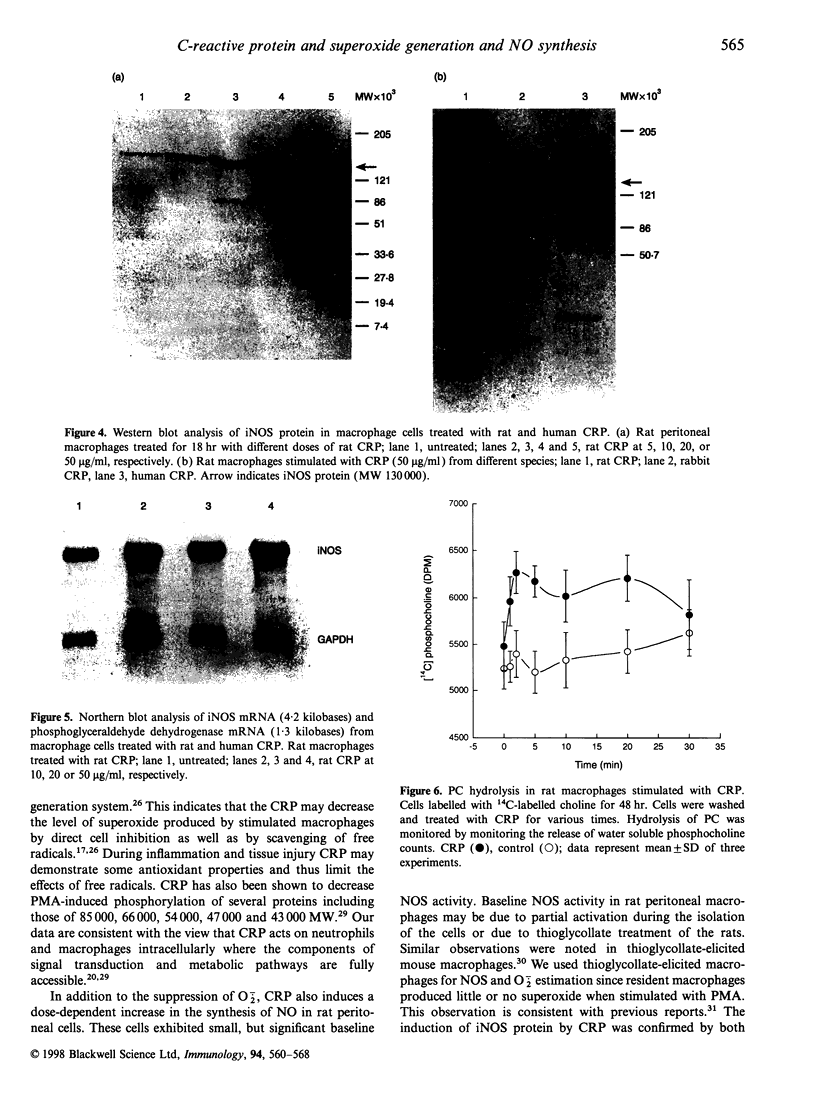


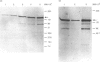
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcoleo F., Milano S., D'Agostino P., Misiano G., Cappelletti S., Gromo G., Marcucci F., Leoni F., Cillari E. Effect of partially modified retro-inverso analogues derived from C-reactive protein on the induction of nitric oxide synthesis in peritoneal macrophages. Br J Pharmacol. 1997 Apr;120(7):1383–1389. doi: 10.1038/sj.bjp.0701050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barna B. P., Deodhar S. D., Gautam S., Yen-Lieberman B., Roberts D. Macrophage activation and generation of tumoricidal activity by liposome-associated human C-reactive protein. Cancer Res. 1984 Jan;44(1):305–310. [PubMed] [Google Scholar]
- Bastian N. R., Hibbs J. B., Jr Assembly and regulation of NADPH oxidase and nitric oxide synthase. Curr Opin Immunol. 1994 Feb;6(1):131–139. doi: 10.1016/0952-7915(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Baumann H., Gauldie J. The acute phase response. Immunol Today. 1994 Feb;15(2):74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchta R., Fridkin M., Pontet M., Contessi E., Scaggiante B., Romeo D. Modulation of human neutrophil function by C-reactive protein. Eur J Biochem. 1987 Feb 16;163(1):141–146. doi: 10.1111/j.1432-1033.1987.tb10747.x. [DOI] [PubMed] [Google Scholar]
- Buchta R., Gennaro R., Pontet M., Fridkin M., Romeo D. C-reactive protein decreases protein phosphorylation in stimulated human neutrophils. FEBS Lett. 1988 Sep 12;237(1-2):173–177. doi: 10.1016/0014-5793(88)80195-9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cillari E., Arcoleo F., Dieli M., D'Agostino R., Gromo G., Leoni F., Milano S. The macrophage-activating tetrapeptide tuftsin induces nitric oxide synthesis and stimulates murine macrophages to kill Leishmania parasites in vitro. Infect Immun. 1994 Jun;62(6):2649–2652. doi: 10.1128/iai.62.6.2649-2652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Dobrinich R., Spagnuolo P. J. Binding of C-reactive protein to human neutrophils. Inhibition of respiratory burst activity. Arthritis Rheum. 1991 Aug;34(8):1031–1038. doi: 10.1002/art.1780340813. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986 May 15;247(1):1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Gewurz H., Mold C., Siegel J., Fiedel B. C-reactive protein and the acute phase response. Adv Intern Med. 1982;27:345–372. [PubMed] [Google Scholar]
- Harbrecht B. G., Billiar T. R., Stadler J., Demetris A. J., Ochoa J., Curran R. D., Simmons R. L. Inhibition of nitric oxide synthesis during endotoxemia promotes intrahepatic thrombosis and an oxygen radical-mediated hepatic injury. J Leukoc Biol. 1992 Oct;52(4):390–394. doi: 10.1002/jlb.52.4.390. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUSHNER I., RAKITA L., KAPLAN M. H. Studies of acute-phase protein. II. Localization of Cx-reactive protein in heart in induced myocardial infarction in rabbits. J Clin Invest. 1963 Feb;42:286–292. doi: 10.1172/JCI104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. H., Volanakis J. E. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974 Jun;112(6):2135–2147. [PubMed] [Google Scholar]
- Kengatharan M., De Kimpe S. J., Thiemermann C. Analysis of the signal transduction in the induction of nitric oxide synthase by lipoteichoic acid in macrophages. Br J Pharmacol. 1996 Mar;117(6):1163–1170. doi: 10.1111/j.1476-5381.1996.tb16711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S., Bhutani L. K., Rao D. N. Release of reactive nitrogen intermediates from the peripheral blood-derived monocytes/macrophages of leprosy patients stimulated in vitro by tuftsin. Lepr Rev. 1997 Mar;68(1):16–24. [PubMed] [Google Scholar]
- Kilpatrick J. M., Volanakis J. E. Molecular genetics, structure, and function of C-reactive protein. Immunol Res. 1991;10(1):43–53. doi: 10.1007/BF02918166. [DOI] [PubMed] [Google Scholar]
- Kushner I., Gewurz H., Benson M. D. C-reactive protein and the acute-phase response. J Lab Clin Med. 1981 Jun;97(6):739–749. [PubMed] [Google Scholar]
- Martin J. H., Edwards S. W. Changes in mechanisms of monocyte/macrophage-mediated cytotoxicity during culture. Reactive oxygen intermediates are involved in monocyte-mediated cytotoxicity, whereas reactive nitrogen intermediates are employed by macrophages in tumor cell killing. J Immunol. 1993 Apr 15;150(8 Pt 1):3478–3486. [PubMed] [Google Scholar]
- Mookerjea S., Hunt D. A novel phosphatidylcholine hydrolysing action of C-reactive protein. Biochem Biophys Res Commun. 1995 Mar 28;208(3):1046–1052. doi: 10.1006/bbrc.1995.1440. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F., Duszkiewicz J. A. Mediation of CRP-dependent phagocytosis through mouse macrophage Fc-receptors. J Immunol. 1977 Nov;119(5):1611–1616. [PubMed] [Google Scholar]
- Müller-Decker K. Interruption of TPA-induced signals by an antiviral and antitumoral xanthate compound: inhibition of a phospholipase C-type reaction. Biochem Biophys Res Commun. 1989 Jul 14;162(1):198–205. doi: 10.1016/0006-291x(89)91981-5. [DOI] [PubMed] [Google Scholar]
- Nagpurkar A., Hunt D., Yang C. Y., Mookerjea S. Degradation of rat C-reactive protein by macrophages. Biochem J. 1993 Oct 1;295(Pt 1):247–253. doi: 10.1042/bj2950247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpurkar A., Mookerjea S. A novel phosphorylcholine-binding protein from rat serum and its effect on heparin-lipoprotein complex formation in the presence of calcium. J Biol Chem. 1981 Jul 25;256(14):7440–7446. [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Novogrodsky A., Vanichkin A., Patya M., Gazit A., Osherov N., Levitzki A. Prevention of lipopolysaccharide-induced lethal toxicity by tyrosine kinase inhibitors. Science. 1994 May 27;264(5163):1319–1322. doi: 10.1126/science.8191285. [DOI] [PubMed] [Google Scholar]
- Rassouli M., Sambasivam H., Azadi P., Dell A., Morris H. R., Nagpurkar A., Mookerjea S., Murray R. K. Derivation of the amino acid sequence of rat C-reactive protein from cDNA cloning with additional studies on the nature of its dimeric component. J Biol Chem. 1992 Feb 15;267(5):2947–2954. [PubMed] [Google Scholar]
- Rosen G. M., Pou S., Ramos C. L., Cohen M. S., Britigan B. E. Free radicals and phagocytic cells. FASEB J. 1995 Feb;9(2):200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- Sambasivam H., Rassouli M., Murray R. K., Nagpurkar A., Mookerjea S., Azadi P., Dell A., Morris H. R. Studies on the carbohydrate moiety and on the biosynthesis of rat C-reactive protein. J Biol Chem. 1993 May 15;268(14):10007–10016. [PubMed] [Google Scholar]
- Schütze S., Potthoff K., Machleidt T., Berkovic D., Wiegmann K., Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992 Nov 27;71(5):765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- Shephard E. G., Anderson R., Rosen O., Myer M. S., Fridkin M., Strachan A. F., De Beer F. C. Peptides generated from C-reactive protein by a neutrophil membrane protease. Amino acid sequence and effects of peptides on neutrophil oxidative metabolism and chemotaxis. J Immunol. 1990 Sep 1;145(5):1469–1476. [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebo J. M., Mortensen R. F. Characterization and isolation of a C-reactive protein receptor from the human monocytic cell line U-937. J Immunol. 1990 Jan 1;144(1):231–238. [PubMed] [Google Scholar]
- Tebo J. M., Mortensen R. F. Internalization and degradation of receptor bound C-reactive protein by U-937 cells: induction of H2O2 production and tumoricidal activity. Biochim Biophys Acta. 1991 Nov 12;1095(3):210–216. doi: 10.1016/0167-4889(91)90101-3. [DOI] [PubMed] [Google Scholar]
- Traynor A. E., Weitzman S. A., Gordon L. I. Bacterial phosphatidylcholine-preferring phospholipase C reversibly inhibits the membrane component of the NADPH oxidase in human polymorphonuclear leukocytes: implications for host defense. Cell Immunol. 1993 Dec;152(2):582–593. doi: 10.1006/cimm.1993.1314. [DOI] [PubMed] [Google Scholar]
- Tschaikowsky K., Meisner M., Schönhuber F., Rügheimer E. Induction of nitric oxide synthase activity in phagocytic cells inhibited by tricyclodecan-9-yl-xanthogenate (D609). Br J Pharmacol. 1994 Nov;113(3):664–668. doi: 10.1111/j.1476-5381.1994.tb17043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volanakis J. E., Kaplan M. H. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc Soc Exp Biol Med. 1971 Feb;136(2):612–614. doi: 10.3181/00379727-136-35323. [DOI] [PubMed] [Google Scholar]
- Zhu L., Gunn C., Beckman J. S. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992 Nov 1;298(2):452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]