Abstract
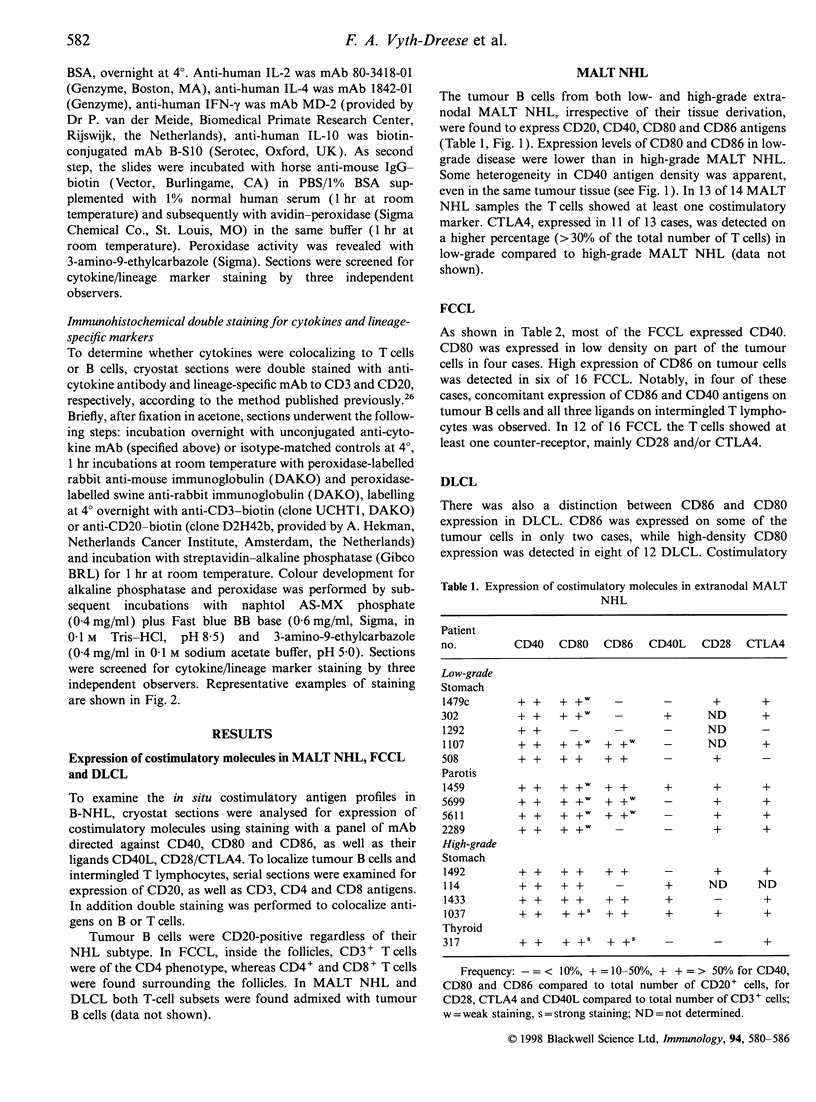
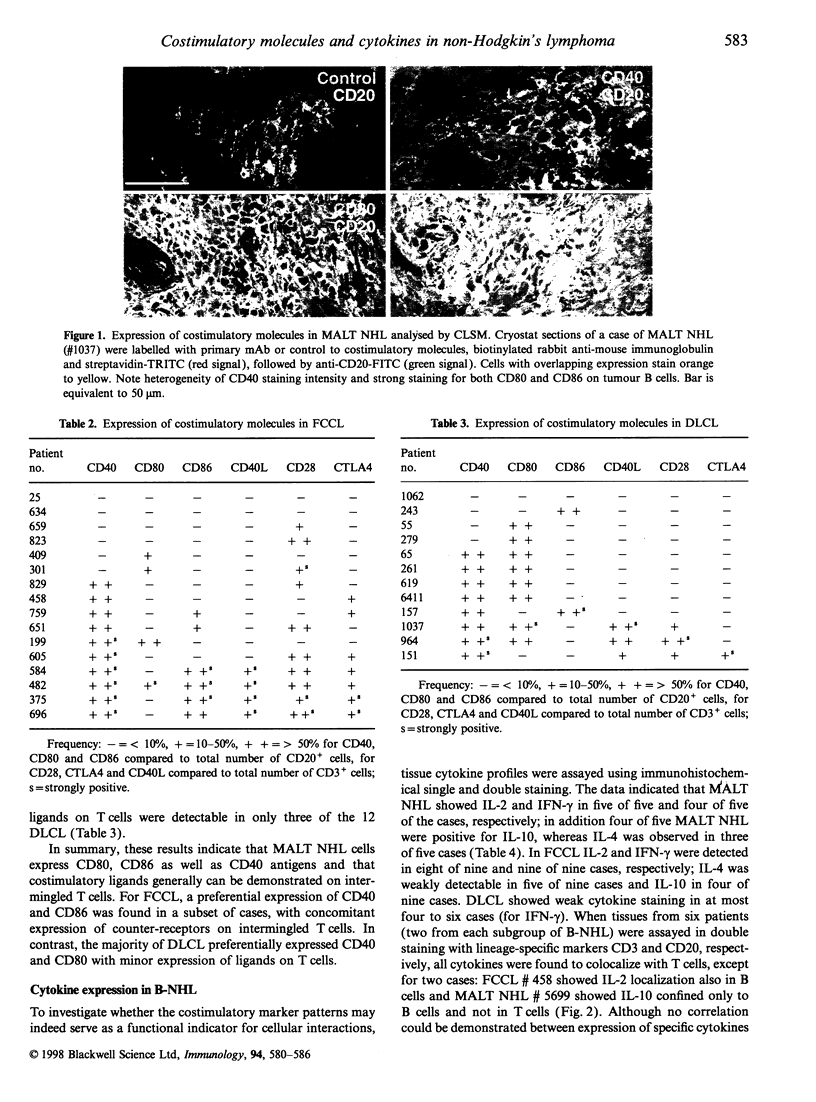
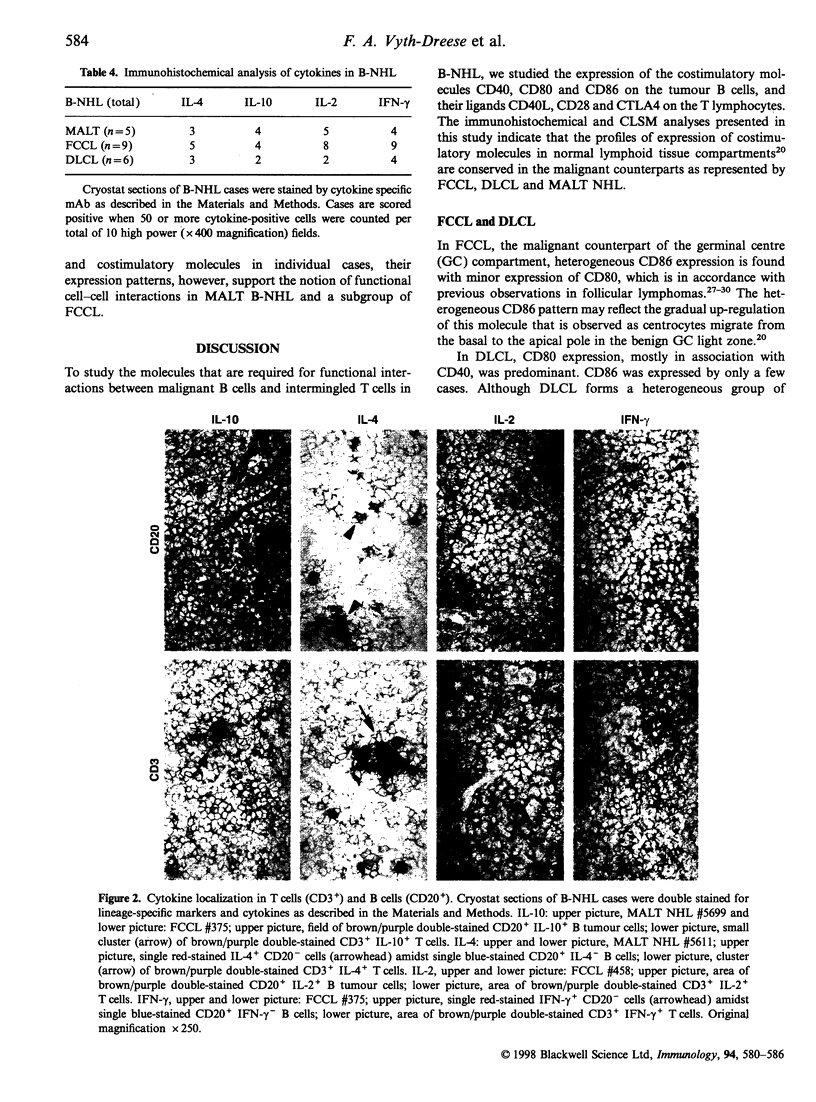
Costimulatory molecules are essential in cognate interactions between T and B lymphocytes. To study the prerequisites of functional interactions between malignant B cells and intermingled T cells in B-cell non-Hodgkin's lymphomas (B-NHL), we examined the expression of CD40, CD80 and CD86 and their ligands CD40 ligand (CD40L, CD154), CD28 and CTLA4 (CD152) using immunohistochemistry and confocal laser scanning microscopy. Almost all mucosa-associated lymphoid tissue (MALT) NHL were positive for CD40 and CD80 and in nine out of 14 cases were positive for CD86. The majority of follicle centre cell lymphomas (FCCL) expressed CD40, but were heterogeneous in their expression of CD80 and CD86. Most diffuse large cell lymphomas (DLCL) were CD80+, but lacked expression of CD86. These patterns reflect the differences in phenotype of normal marginal-zone B cells (as counterparts of MALT NHL) and germinal centre cells (as counterparts of FCCL and DLCL). Counter-receptors on T cells were detectable in 13 of 14 MALT NHL, 12 of 16 FCCL but only occasionally in DLCL (three of 12 cases). A subgroup of FCCL was identified with T-cell expression of CD40L, CD28 and CTLA4 simultaneously with strong expression of CD40 and CD86 on the tumour B cells. These results indicate that MALT NHL and a subset of FCCL are most optimally equipped for functional interactions with T cells. This may be supported by the demonstration of cytokine production - mainly in T cells - in MALT NHL [interleukin-2 (IL-2), interferon-gamma (IFN-gamma), IL-10] and FCCL (IL-2, IFN-gamma) and to a lesser extent in DLCL.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arpin C., Déchanet J., Van Kooten C., Merville P., Grouard G., Brière F., Banchereau J., Liu Y. J. Generation of memory B cells and plasma cells in vitro. Science. 1995 May 5;268(5211):720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Bazan F., Blanchard D., Brière F., Galizzi J. P., van Kooten C., Liu Y. J., Rousset F., Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Bayerdörffer E., Neubauer A., Rudolph B., Thiede C., Lehn N., Eidt S., Stolte M. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995 Jun 24;345(8965):1591–1594. doi: 10.1016/s0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]
- Boot H., de Jong D., van Heerde P., Taal B. Role of Helicobacter pylori eradication in high-grade MALT lymphoma. Lancet. 1995 Aug 12;346(8972):448–449. doi: 10.1016/s0140-6736(95)92823-5. [DOI] [PubMed] [Google Scholar]
- Carbone A., Gloghini A., Gruss H. J., Pinto A. CD40 ligand is constitutively expressed in a subset of T cell lymphomas and on the microenvironmental reactive T cells of follicular lymphomas and Hodgkin's disease. Am J Pathol. 1995 Oct;147(4):912–922. [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. How B and T cells talk to each other. Nature. 1994 Feb 3;367(6462):425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Spencer J. Immunologic aspects of Helicobacter pylori infection and malignant transformation of B cells. Semin Gastrointest Dis. 1996 Jan;7(1):30–40. [PubMed] [Google Scholar]
- Delabie J., Ceuppens J. L., Vandenberghe P., de Boer M., Coorevits L., De Wolf-Peeters C. The B7/BB1 antigen is expressed by Reed-Sternberg cells of Hodgkin's disease and contributes to the stimulating capacity of Hodgkin's disease-derived cell lines. Blood. 1993 Nov 1;82(9):2845–2852. [PubMed] [Google Scholar]
- Freeman G. J., Borriello F., Hodes R. J., Reiser H., Hathcock K. S., Laszlo G., McKnight A. J., Kim J., Du L., Lombard D. B. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science. 1993 Nov 5;262(5135):907–909. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Freedman A. S., Segil J. M., Lee G., Whitman J. F., Nadler L. M. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1989 Oct 15;143(8):2714–2722. [PubMed] [Google Scholar]
- Genta R. M., Hamner H. W., Graham D. Y. Gastric lymphoid follicles in Helicobacter pylori infection: frequency, distribution, and response to triple therapy. Hum Pathol. 1993 Jun;24(6):577–583. doi: 10.1016/0046-8177(93)90235-9. [DOI] [PubMed] [Google Scholar]
- Greiner A., Knörr C., Qin Y., Sebald W., Schimpl A., Banchereau J., Müller-Hermelink H. K. Low-grade B cell lymphomas of mucosa-associated lymphoid tissue (MALT-type) require CD40-mediated signaling and Th2-type cytokines for in vitro growth and differentiation. Am J Pathol. 1997 May;150(5):1583–1593. [PMC free article] [PubMed] [Google Scholar]
- Hussell T., Isaacson P. G., Crabtree J. E., Spencer J. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol. 1996 Feb;178(2):122–127. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Hussell T., Isaacson P. G., Crabtree J. E., Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet. 1993 Sep 4;342(8871):571–574. doi: 10.1016/0140-6736(93)91408-e. [DOI] [PubMed] [Google Scholar]
- Isaacson P. G. Extranodal lymphomas: the MALT concept. Verh Dtsch Ges Pathol. 1992;76:14–23. [PubMed] [Google Scholar]
- Jacob M. C., Piccinni M. P., Bonnefoix T., Sotto M. F., Couderc P., Bensa J. C., Sotto J. J. T lymphocytes from invaded lymph nodes in patients with B-cell-derived non-Hodgkin's lymphoma: reactivity toward the malignant clone. Blood. 1990 Mar 1;75(5):1154–1162. [PubMed] [Google Scholar]
- June C. H., Bluestone J. A., Nadler L. M., Thompson C. B. The B7 and CD28 receptor families. Immunol Today. 1994 Jul;15(7):321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Koulis A., Diss T., Isaacson P. G., Dogan A. Characterization of tumor-infiltrating T lymphocytes in B-cell lymphomas of mucosa-associated lymphoid tissue. Am J Pathol. 1997 Nov;151(5):1353–1360. [PMC free article] [PubMed] [Google Scholar]
- Laman J. D., Gerritse K., Fasbender M., Boersma W. J., van Rooijen N., Claassen E. Double immunocytochemical staining for in vivo detection of epitope specificity and isotype of antibody-forming cells against synthetic peptides homologous to human immunodeficiency virus-1. J Histochem Cytochem. 1990 Apr;38(4):457–462. doi: 10.1177/38.4.1690764. [DOI] [PubMed] [Google Scholar]
- Lederman S., Yellin M. J., Krichevsky A., Belko J., Lee J. J., Chess L. Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help). J Exp Med. 1992 Apr 1;175(4):1091–1101. doi: 10.1084/jem.175.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D. J., Walunas T. L., Bluestone J. A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Greene J. L., Tan P., Bradshaw J., Ledbetter J. A., Anasetti C., Damle N. K. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992 Dec 1;176(6):1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. J., Barthélémy C., de Bouteiller O., Arpin C., Durand I., Banchereau J. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7-1 and B7-2. Immunity. 1995 Mar;2(3):239–248. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Munro J. M., Freedman A. S., Aster J. C., Gribben J. G., Lee N. C., Rhynhart K. K., Banchereau J., Nadler L. M. In vivo expression of the B7 costimulatory molecule by subsets of antigen-presenting cells and the malignant cells of Hodgkin's disease. Blood. 1994 Feb 1;83(3):793–798. [PubMed] [Google Scholar]
- Nelson E. L., Li X., Hsu F. J., Kwak L. W., Levy R., Clayberger C., Krensky A. M. Tumor-specific, cytotoxic T-lymphocyte response after idiotype vaccination for B-cell, non-Hodgkin's lymphoma. Blood. 1996 Jul 15;88(2):580–589. [PubMed] [Google Scholar]
- Roggero E., Zucca E., Pinotti G., Pascarella A., Capella C., Savio A., Pedrinis E., Paterlini A., Venco A., Cavalli F. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med. 1995 May 15;122(10):767–769. doi: 10.7326/0003-4819-122-10-199505150-00006. [DOI] [PubMed] [Google Scholar]
- Schultze J. L., Cardoso A. A., Freeman G. J., Seamon M. J., Daley J., Pinkus G. S., Gribben J. G., Nadler L. M. Follicular lymphomas can be induced to present alloantigen efficiently: a conceptual model to improve their tumor immunogenicity. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8200–8204. doi: 10.1073/pnas.92.18.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze J. L., Seamon M. J., Michalak S., Gribben J. G., Nadler L. M. Autologous tumor infiltrating T cells cytotoxic for follicular lymphoma cells can be expanded in vitro. Blood. 1997 May 15;89(10):3806–3816. [PubMed] [Google Scholar]
- Shi I., Bonnefoix T., Heuzé-Le Vacon F., Jacob M. C., Leroux D., Gressin R., Sotto M. F., Chaffanjon P., Bensa J. C., Sotto J. J. Autotumour reactive T-cell clones among tumour-infiltrating T lymphocytes in B-cell non-Hodgkin's lymphomas. Br J Haematol. 1995 Aug;90(4):837–843. doi: 10.1111/j.1365-2141.1995.tb05204.x. [DOI] [PubMed] [Google Scholar]
- Vallé A., Zuber C. E., Defrance T., Djossou O., De Rie M., Banchereau J. Activation of human B lymphocytes through CD40 and interleukin 4. Eur J Immunol. 1989 Aug;19(8):1463–1467. doi: 10.1002/eji.1830190818. [DOI] [PubMed] [Google Scholar]
- Vyth-Dreese F. A., Dellemijn T. A., Majoor D., de Jong D. Localization in situ of the co-stimulatory molecules B7.1, B7.2, CD40 and their ligands in normal human lymphoid tissue. Eur J Immunol. 1995 Nov;25(11):3023–3029. doi: 10.1002/eji.1830251106. [DOI] [PubMed] [Google Scholar]
- Vyth-Dreese F. A., Dellemijn T. A., van Oostveen J. W., Feltkamp C. A., Hekman A. Functional expression of adhesion receptors and costimulatory molecules by fresh and immortalized B-cell non-Hodgkin's lymphoma cells. Blood. 1995 May 15;85(10):2802–2812. [PubMed] [Google Scholar]
- Wotherspoon A. C., Doglioni C., Diss T. C., Pan L., Moschini A., de Boni M., Isaacson P. G. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993 Sep 4;342(8871):575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- de Boer M., Parren P., Dove J., Ossendorp F., van der Horst G., Reeder J. Functional characterization of a novel anti-B7 monoclonal antibody. Eur J Immunol. 1992 Dec;22(12):3071–3075. doi: 10.1002/eji.1830221207. [DOI] [PubMed] [Google Scholar]




