Abstract
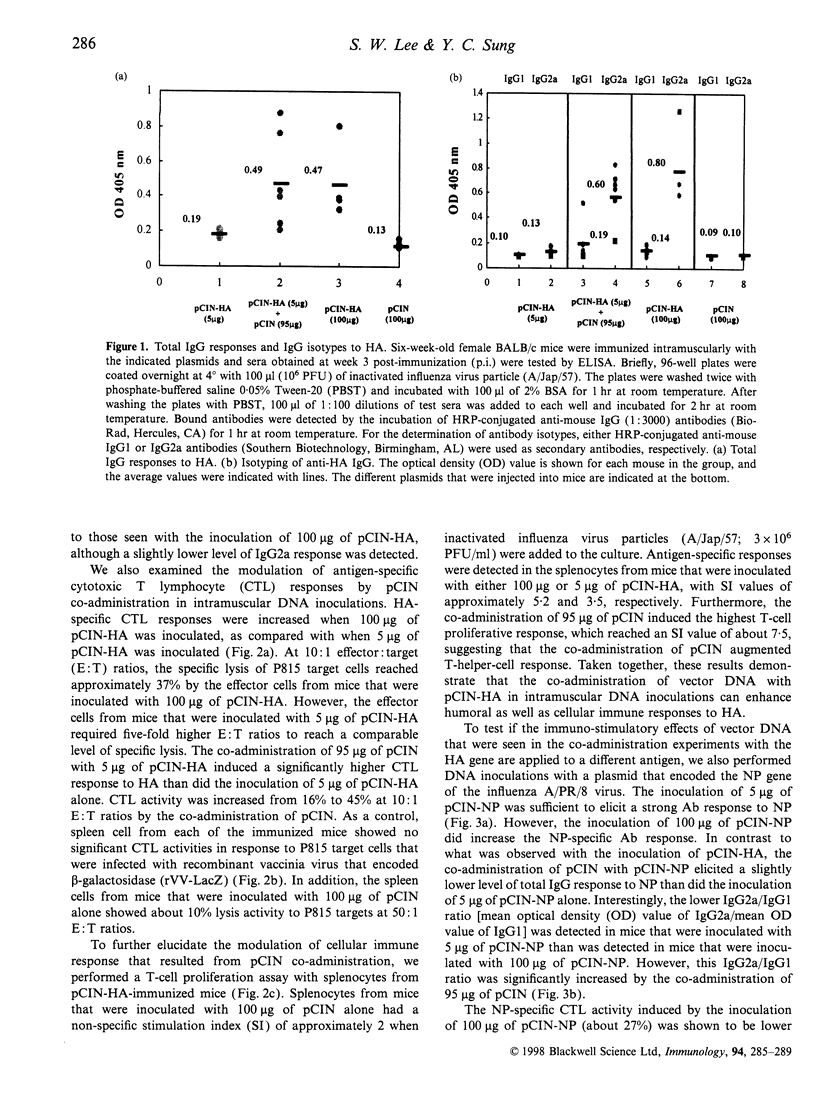
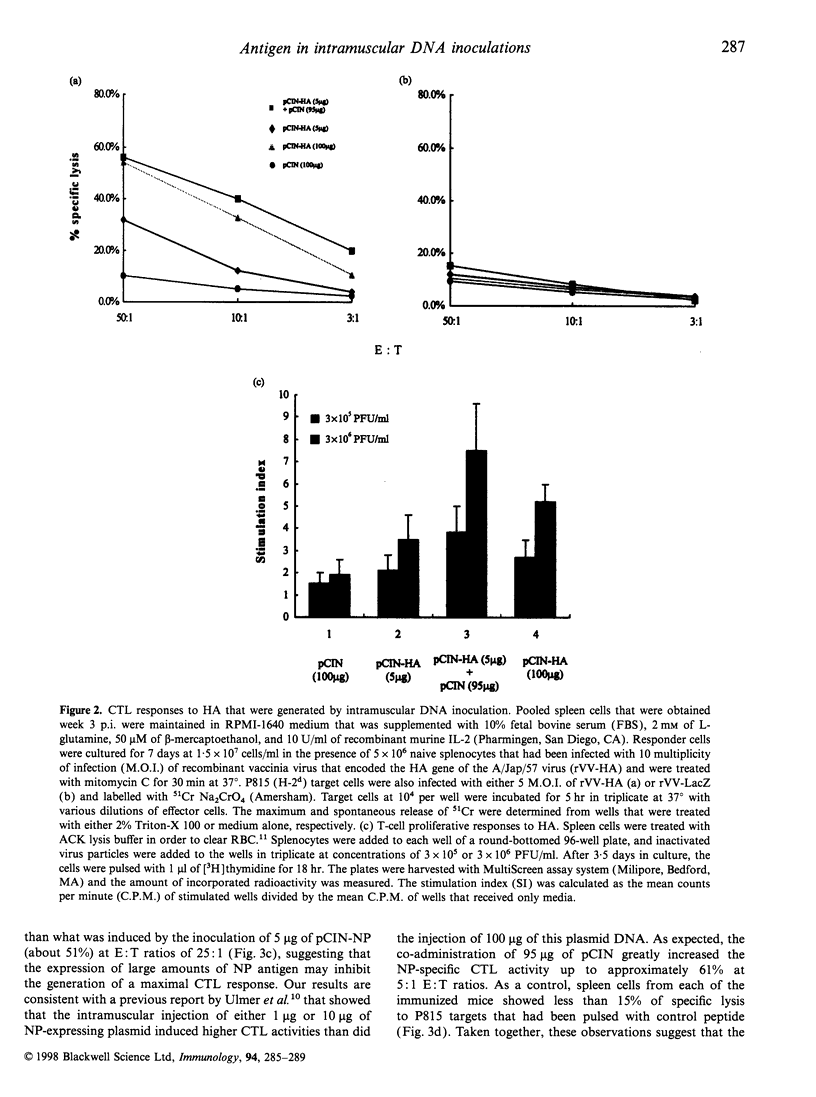
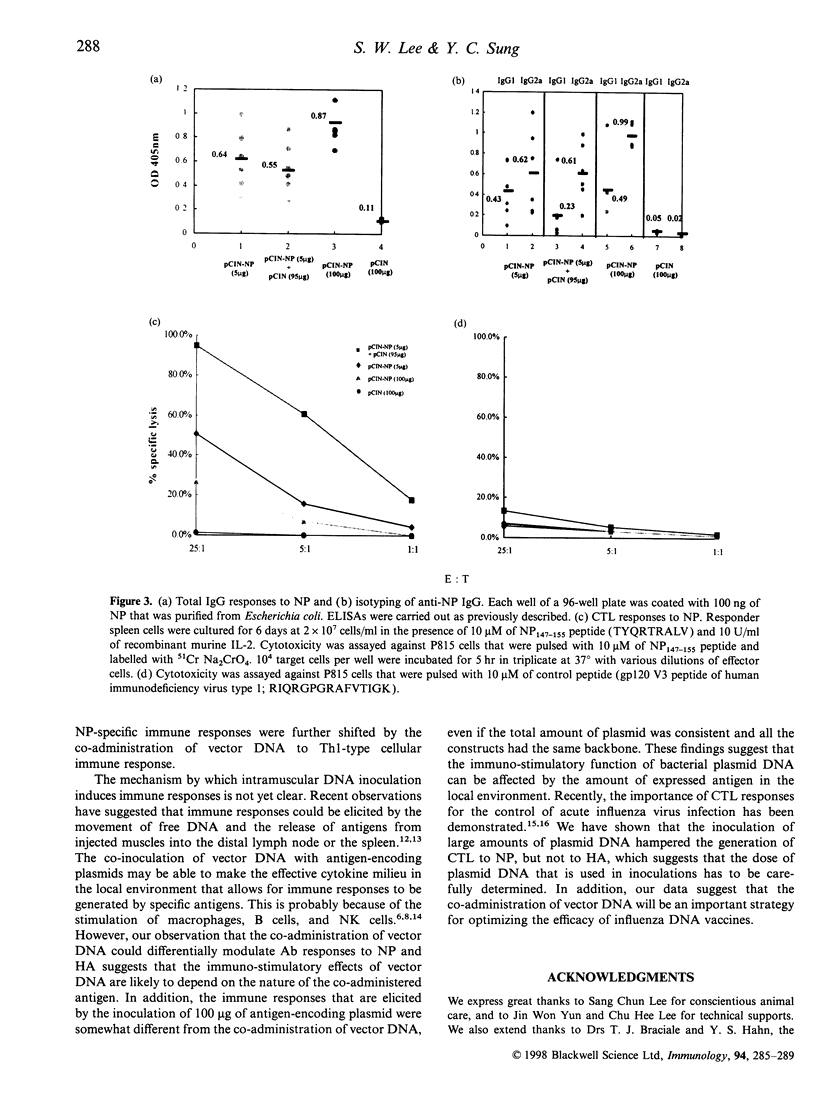
The CpG motifs of bacterial-derived plasmids augment antigen-specific immune responses and steer those responses towards the T helper 1 (Th1) type. In this study, we have addressed the immuno-stimulatory effect of intramuscular co-administration of CpG motifs containing vector DNA on the modulation of immune responses to the haemagglutinin (HA) and the nucleoprotein (NP) proteins of influenza virus. The co-administration of vector DNA with a HA-encoding plasmid DNA showed a significant enhancement in the total IgG response, the generation of cytotoxic T lymphocyte (CTL), and the T-cell proliferative response. In the case of NP-encoding plasmid DNA inoculations, the co-administration of vector DNA slightly decreased the total IgG response, although the IgG2a/IgG1 ratio and the CTL responses to NP were significantly increased. These observations suggest that the immuno-stimulatory effects of bacterial-derived plasmids depend upon the nature of the co-administered antigen.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu R. S., Targoni O. S., Krieg A. M., Lehmann P. V., Harding C. V. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997 Nov 17;186(10):1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C. Cytotoxic T cell effector and memory function in viral immunity. Curr Top Microbiol Immunol. 1996;206:1–14. doi: 10.1007/978-3-642-85208-4_1. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Topham D. J., Tripp R. A., Cardin R. D., Brooks J. W., Stevenson P. G. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997 Oct;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Feltquate D. M., Heaney S., Webster R. G., Robinson H. L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997 Mar 1;158(5):2278–2284. [PubMed] [Google Scholar]
- Klinman D. M., Yi A. K., Beaucage S. L., Conover J., Krieg A. M. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci U S A. 1996 Apr 2;93(7):2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg A. M., Yi A. K., Matson S., Waldschmidt T. J., Bishop G. A., Teasdale R., Koretzky G. A., Klinman D. M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995 Apr 6;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- Pertmer T. M., Roberts T. R., Haynes J. R. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996 Sep;70(9):6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Torres C. A. DNA vaccines. Semin Immunol. 1997 Oct;9(5):271–283. doi: 10.1006/smim.1997.0083. [DOI] [PubMed] [Google Scholar]
- Roman M., Martin-Orozco E., Goodman J. S., Nguyen M. D., Sato Y., Ronaghy A., Kornbluth R. S., Richman D. D., Carson D. A., Raz E. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997 Aug;3(8):849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- Tang D. C., DeVit M., Johnston S. A. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992 Mar 12;356(6365):152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- Torres C. A., Iwasaki A., Barber B. H., Robinson H. L. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997 May 15;158(10):4529–4532. [PubMed] [Google Scholar]
- Ulmer J. B., Deck R. R., DeWitt C. M., Friedman A., Donnelly J. J., Liu M. A. Protective immunity by intramuscular injection of low doses of influenza virus DNA vaccines. Vaccine. 1994 Dec;12(16):1541–1544. doi: 10.1016/0264-410x(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Ulmer J. B., Sadoff J. C., Liu M. A. DNA vaccines. Curr Opin Immunol. 1996 Aug;8(4):531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- Whalen R. G. DNA vaccines for emerging infectious diseases: what if? Emerg Infect Dis. 1996 Jul-Sep;2(3):168–175. doi: 10.3201/eid0203.960302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]