Abstract
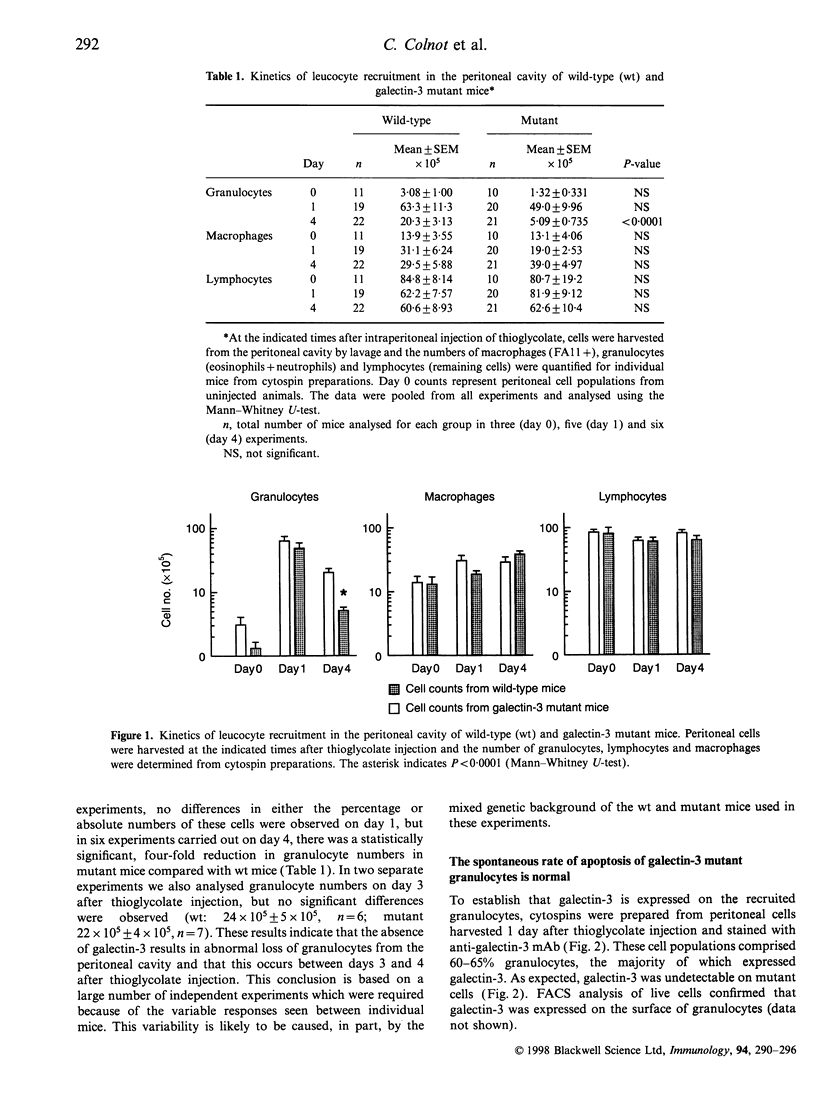
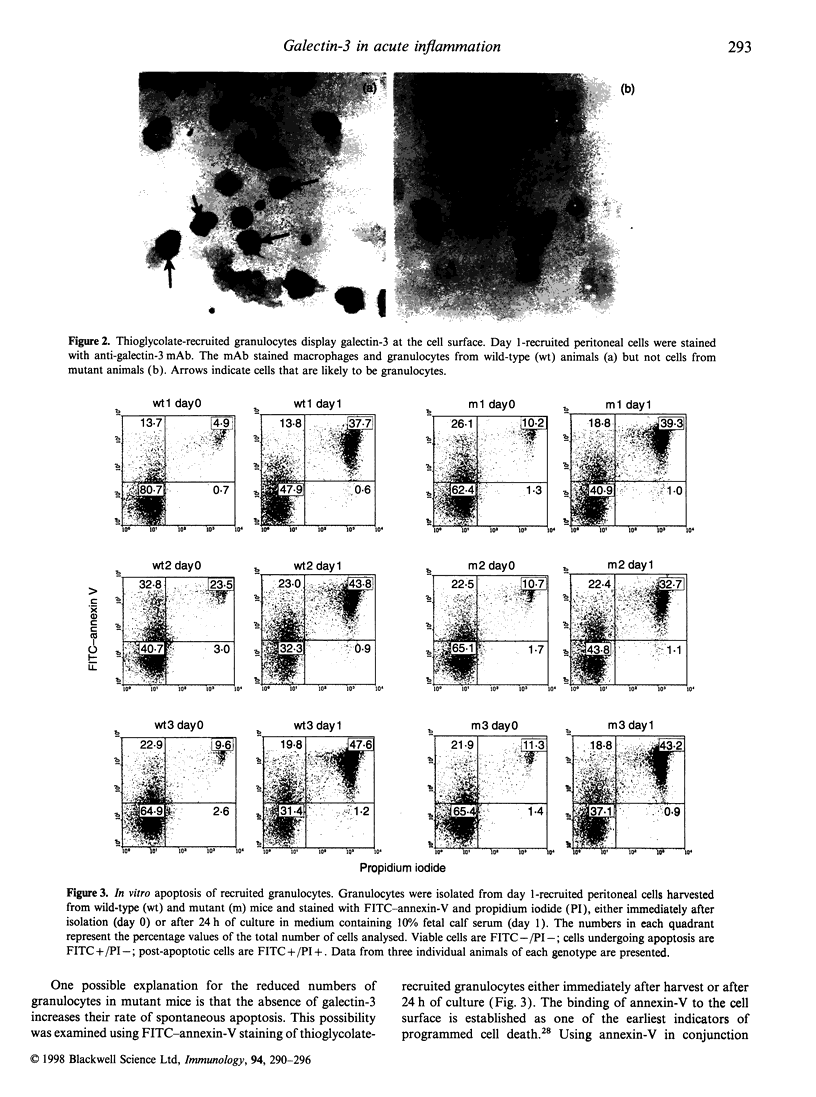
Galectin-3, also known as the macrophage marker Mac-2, is a member of a family of structurally related animal lectins that exhibit specificity for beta-galactosides. In order to investigate the role of galectin-3 in acute inflammation, we have compared the number of leucocytes present in the peritoneal cavity of wild type and galectin-3 null mutant mice after intraperitoneal (i.p.) injection of thioglycolate broth. At day 1 after injection, we found no difference in the recruitment of mononuclear phagocytes and granulocytes to the peritoneal cavity. However, 4 days after thioglycolate injection, galectin-3 mutant mice exhibited a significantly reduced number of recoverable granulocytes compared to wild-type animals. As mutant granulocytes did not exhibit an accelerated rate of apoptosis and their uptake by macrophages appeared to be unaffected by the mutation, the phenotype described here suggests that galectin-3 participates in an additional level of control during the resolution of acute inflammation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellingan G. J., Caldwell H., Howie S. E., Dransfield I., Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996 Sep 15;157(6):2577–2585. [PubMed] [Google Scholar]
- Benimetskaya L., Loike J. D., Khaled Z., Loike G., Silverstein S. C., Cao L., el Khoury J., Cai T. Q., Stein C. A. Mac-1 (CD11b/CD18) is an oligodeoxynucleotide-binding protein. Nat Med. 1997 Apr;3(4):414–420. doi: 10.1038/nm0497-414. [DOI] [PubMed] [Google Scholar]
- Cherayil B. J., Weiner S. J., Pillai S. The Mac-2 antigen is a galactose-specific lectin that binds IgE. J Exp Med. 1989 Dec 1;170(6):1959–1972. doi: 10.1084/jem.170.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot C., Fowlis D., Ripoche M. A., Bouchaert I., Poirier F. Embryonic implantation in galectin 1/galectin 3 double mutant mice. Dev Dyn. 1998 Apr;211(4):306–313. doi: 10.1002/(SICI)1097-0177(199804)211:4<306::AID-AJA2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Massa S. M., Barondes S. H. Endogenous muscle lectin inhibits myoblast adhesion to laminin. J Cell Biol. 1991 Dec;115(5):1437–1448. doi: 10.1083/jcb.115.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon A., Rieu P., Barkalow F. J., Askari S., Sharpe A. H., von Andrian U. H., Arnaout M. A., Mayadas T. N. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996 Dec;5(6):653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- Dagher S. F., Wang J. L., Patterson R. J. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1213–1217. doi: 10.1073/pnas.92.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S., Hughes R. C. Macrophage surface glycoproteins binding to galectin-3 (Mac-2-antigen). Glycoconj J. 1997 Feb;14(2):267–274. doi: 10.1023/a:1018554124545. [DOI] [PubMed] [Google Scholar]
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988 Jul 15;263(20):9557–9560. [PubMed] [Google Scholar]
- Frigeri L. G., Liu F. T. Surface expression of functional IgE binding protein, an endogenous lectin, on mast cells and macrophages. J Immunol. 1992 Feb 1;148(3):861–867. [PubMed] [Google Scholar]
- Frigeri L. G., Zuberi R. I., Liu F. T. Epsilon BP, a beta-galactoside-binding animal lectin, recognizes IgE receptor (Fc epsilon RI) and activates mast cells. Biochemistry. 1993 Aug 3;32(30):7644–7649. doi: 10.1021/bi00081a007. [DOI] [PubMed] [Google Scholar]
- Ho M. K., Springer T. A. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982 Mar;128(3):1221–1228. [PubMed] [Google Scholar]
- Hughes J., Johnson R. J., Mooney A., Hugo C., Gordon K., Savill J. Neutrophil fate in experimental glomerular capillary injury in the rat. Emigration exceeds in situ clearance by apoptosis. Am J Pathol. 1997 Jan;150(1):223–234. [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C. Mac-2: a versatile galactose-binding protein of mammalian tissues. Glycobiology. 1994 Feb;4(1):5–12. doi: 10.1093/glycob/4.1.5. [DOI] [PubMed] [Google Scholar]
- Kuwabara I., Liu F. T. Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol. 1996 May 15;156(10):3939–3944. [PubMed] [Google Scholar]
- Lagasse E., Weissman I. L. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994 Mar 1;179(3):1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. T., Hsu D. K., Zuberi R. I., Kuwabara I., Chi E. Y., Henderson W. R., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995 Oct;147(4):1016–1028. [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Apicella M. A., Lindstedt R., Leffler H. Possible interaction between animal lectins and bacterial carbohydrates. Methods Enzymol. 1994;236:231–254. doi: 10.1016/0076-6879(94)36019-7. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Reutelingsperger C. P., McGahon A. J., Rader J. A., van Schie R. C., LaFace D. M., Green D. R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995 Nov 1;182(5):1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa S. M., Cooper D. N., Leffler H., Barondes S. H. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry. 1993 Jan 12;32(1):260–267. doi: 10.1021/bi00052a033. [DOI] [PubMed] [Google Scholar]
- Mehul B., Bawumia S., Hughes R. C. Cross-linking of galectin 3, a galactose-binding protein of mammalian cells, by tissue-type transglutaminase. FEBS Lett. 1995 Feb 27;360(2):160–164. doi: 10.1016/0014-5793(95)00100-n. [DOI] [PubMed] [Google Scholar]
- Mehul B., Hughes R. C. Plasma membrane targetting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion. J Cell Sci. 1997 May;110(Pt 10):1169–1178. doi: 10.1242/jcs.110.10.1169. [DOI] [PubMed] [Google Scholar]
- Mey A., Leffler H., Hmama Z., Normier G., Revillard J. P. The animal lectin galectin-3 interacts with bacterial lipopolysaccharides via two independent sites. J Immunol. 1996 Feb 15;156(4):1572–1577. [PubMed] [Google Scholar]
- Pearson A. M. Scavenger receptors in innate immunity. Curr Opin Immunol. 1996 Feb;8(1):20–28. doi: 10.1016/s0952-7915(96)80100-2. [DOI] [PubMed] [Google Scholar]
- Rabinowitz S. S., Gordon S. Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli. J Exp Med. 1991 Oct 1;174(4):827–836. doi: 10.1084/jem.174.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Burdett I., Hughes R. C. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex. Exp Cell Res. 1993 Jul;207(1):8–18. doi: 10.1006/excr.1993.1157. [DOI] [PubMed] [Google Scholar]
- Sato S., Hughes R. C. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem. 1994 Feb 11;269(6):4424–4430. [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Fadok V., Henson P., Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993 Mar;14(3):131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Savill J., Hogg N., Ren Y., Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992 Oct;90(4):1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J. Phagocyte recognition of apoptotic cells. Biochem Soc Trans. 1996 Nov;24(4):1065–1069. doi: 10.1042/bst0241065. [DOI] [PubMed] [Google Scholar]
- Truong M. J., Gruart V., Kusnierz J. P., Papin J. P., Loiseau S., Capron A., Capron M. Human neutrophils express immunoglobulin E (IgE)-binding proteins (Mac-2/epsilon BP) of the S-type lectin family: role in IgE-dependent activation. J Exp Med. 1993 Jan 1;177(1):243–248. doi: 10.1084/jem.177.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong M. J., Gruart V., Liu F. T., Prin L., Capron A., Capron M. IgE-binding molecules (Mac-2/epsilon BP) expressed by human eosinophils. Implication in IgE-dependent eosinophil cytotoxicity. Eur J Immunol. 1993 Dec;23(12):3230–3235. doi: 10.1002/eji.1830231228. [DOI] [PubMed] [Google Scholar]
- Wang J. L., Laing J. G., Anderson R. L. Lectins in the cell nucleus. Glycobiology. 1991 Jun;1(3):243–252. doi: 10.1093/glycob/1.3.243. [DOI] [PubMed] [Google Scholar]
- Woo H. J., Shaw L. M., Messier J. M., Mercurio A. M. The major non-integrin laminin binding protein of macrophages is identical to carbohydrate binding protein 35 (Mac-2). J Biol Chem. 1990 May 5;265(13):7097–7099. [PubMed] [Google Scholar]
- Yamaoka A., Kuwabara I., Frigeri L. G., Liu F. T. A human lectin, galectin-3 (epsilon bp/Mac-2), stimulates superoxide production by neutrophils. J Immunol. 1995 Apr 1;154(7):3479–3487. [PubMed] [Google Scholar]
- Yang R. Y., Hsu D. K., Liu F. T. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]



