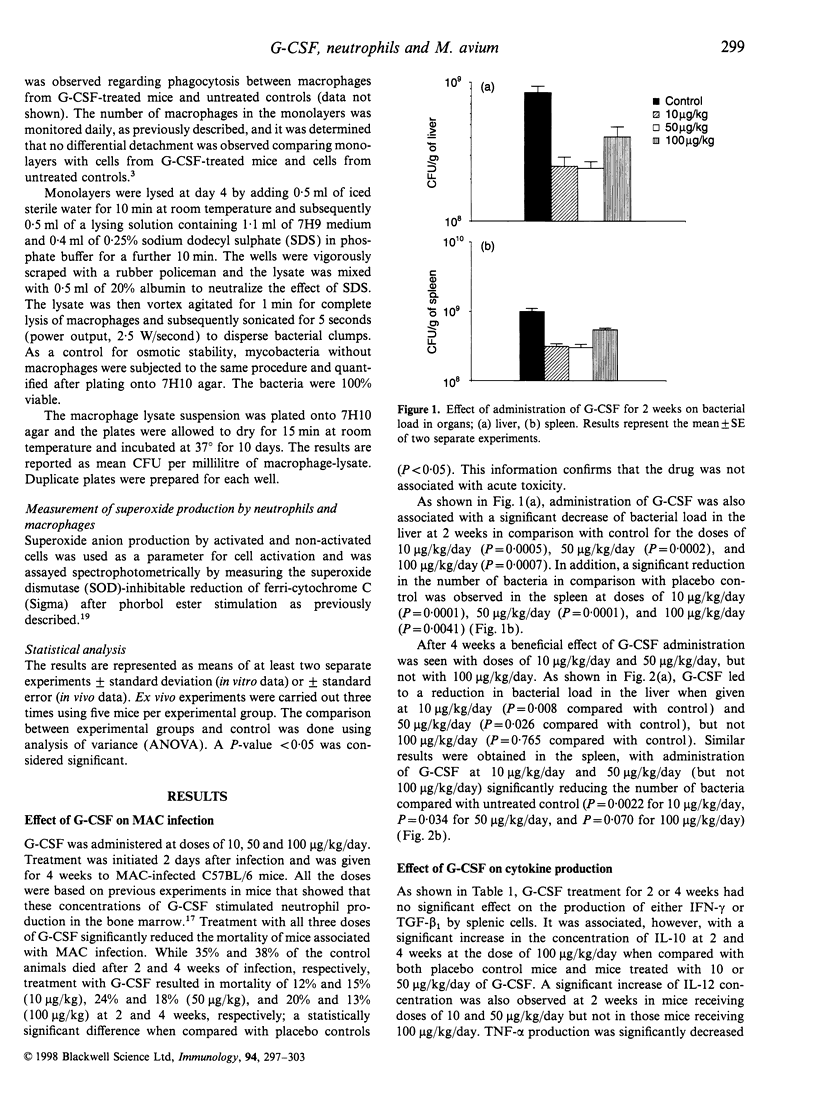
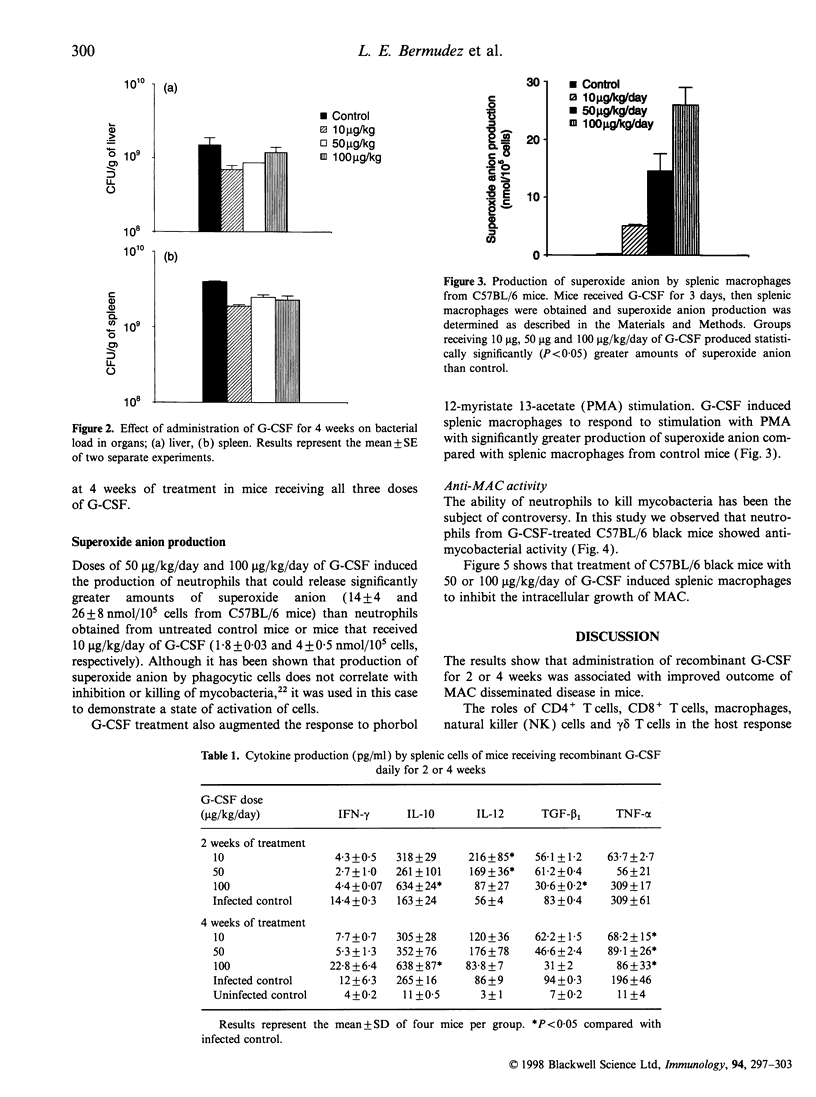
Abstract
A role of neutrophils in the host response against Mycobacterium avium (MAC) has recently been suggested. To investigate this matter further, we determined the effect of granulocyte colony-stimulating factor (G-CSF) on the outcome of MAC infection in mice. C57BL/6bg+/bg- black mice were intravenously infected with 1 x 10(7) MAC and then divided into four experimental groups to receive G-CSF as follows: (i) 10 micrograms/kg/day; (ii) 50 micrograms/kg/day; (iii) 100 micrograms/kg/day; (iv) placebo control. Mice were killed at 2 and 4 weeks of treatment to determine the bacterial load of liver and spleen. Treatment with G-CSF at both 10 and 50 micrograms/kg/day doses significantly decreased the number of viable bacteria in liver and spleen after 2 weeks (approximately 70.5% and 69.0%, respectively), and after 4 weeks (approximately 53% and 52%, respectively, P < 0.05 compared with placebo control). Treatment with 100 micrograms/kg/day did not result in decrease of bacterial colony-forming units in the liver and spleen after 4 weeks. Administration of G-CSF induced interleukin-10 (IL-10) and IL-12 production by splenocytes. To examine if the protective effect of G-CSF was accompanied by the activation of phagocytic cells, blood neutrophils and splenic macrophages were purified from mice receiving G-CSF and their ability to kill MAC was examined ex vivo. Neutrophils and macrophages from G-CSF-treated mice were able to inhibit the growth of or to kill MAC ex vivo, while phagocytic cells from untreated control mice had no anti-MAC effect. These results suggest that activation of neutrophils appears to induce an effective non-specific host defence against MAC, and further studies should aim for better understanding of the mechanisms of protection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg R., Castro A. G., Gomes S., Pedrosa J., Silva M. T. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect Immun. 1995 Sep;63(9):3381–3387. doi: 10.1128/iai.63.9.3381-3387.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Orme I. M. Effector mechanisms involved in cytokine-mediated bacteriostasis of Mycobacterium avium infections in murine macrophages. Immunology. 1993 Nov;80(3):352–359. [PMC free article] [PubMed] [Google Scholar]
- Asano S., Ono M. Human granulocyte colony-stimulating factor: its biological actions and clinical implication. Nihon Ketsueki Gakkai Zasshi. 1987 Dec;50(8):1550–1556. [PubMed] [Google Scholar]
- Bermudez L. E., Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993 Jul;61(7):3093–3097. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E. Infection of "nonprofessional phagocytes" with Mycobacterium avium complex. Clin Immunol Immunopathol. 1991 Nov;61(2 Pt 1):225–235. doi: 10.1016/s0090-1229(05)80026-1. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Martinelli J. C., Gascon R., Wu M., Young L. S. Protection against gram-negative bacteremia in neutropenic mice with recombinant granulocyte-macrophage colony-stimulating factor. Cytokine. 1990 Jul;2(4):287–293. doi: 10.1016/1043-4666(90)90030-w. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Martinelli J., Petrofsky M., Kolonoski P., Young L. S. Recombinant granulocyte-macrophage colony-stimulating factor enhances the effects of antibiotics against Mycobacterium avium complex infection in the beige mouse model. J Infect Dis. 1994 Mar;169(3):575–580. doi: 10.1093/infdis/169.3.575. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Stevens P., Kolonoski P., Wu M., Young L. S. Treatment of experimental disseminated Mycobacterium avium complex infection in mice with recombinant IL-2 and tumor necrosis factor. J Immunol. 1989 Nov 1;143(9):2996–3000. [PubMed] [Google Scholar]
- Bermudez L. E., Wu M., Young L. S. Interleukin-12-stimulated natural killer cells can activate human macrophages to inhibit growth of Mycobacterium avium. Infect Immun. 1995 Oct;63(10):4099–4104. doi: 10.1128/iai.63.10.4099-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Factors affecting invasion of HT-29 and HEp-2 epithelial cells by organisms of the Mycobacterium avium complex. Infect Immun. 1994 May;62(5):2021–2026. doi: 10.1128/iai.62.5.2021-2026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Natural killer cell-dependent mycobacteriostatic and mycobactericidal activity in human macrophages. J Immunol. 1991 Jan 1;146(1):265–270. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Oxidative and non-oxidative intracellular killing of Mycobacterium avium complex. Microb Pathog. 1989 Oct;7(4):289–298. doi: 10.1016/0882-4010(89)90047-8. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Phagocytosis and intracellular killing of Mycobacterium avium complex by human and murine macrophages. Braz J Med Biol Res. 1987;20(2):191–201. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Recombinant granulocyte-macrophage colony-stimulating factor activates human macrophages to inhibit growth or kill Mycobacterium avium complex. J Leukoc Biol. 1990 Jul;48(1):67–73. doi: 10.1002/jlb.48.1.67. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., McMillen S., Hoffman S. L., Djeu J. Y. Mycobacterial induction of activated killer cells: possible role of tyrosine kinase activity in interleukin-2 receptor alpha expression. Infect Immun. 1992 Jul;60(7):2843–2849. doi: 10.1128/iai.60.7.2843-2849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bober L. A., Grace M. J., Pugliese-Sivo C., Rojas-Triana A., Waters T., Sullivan L. M., Narula S. K. The effect of GM-CSF and G-CSF on human neutrophil function. Immunopharmacology. 1995 Mar;29(2):111–119. doi: 10.1016/0162-3109(94)00050-p. [DOI] [PubMed] [Google Scholar]
- Brown A. E., Holzer T. J., Andersen B. R. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J Infect Dis. 1987 Dec;156(6):985–989. doi: 10.1093/infdis/156.6.985. [DOI] [PubMed] [Google Scholar]
- Cassatella M. A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995 Jan;16(1):21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- Champsi J., Young L. S., Bermudez L. E. Production of TNF-alpha, IL-6 and TGF-beta, and expression of receptors for TNF-alpha and IL-6, during murine Mycobacterium avium infection. Immunology. 1995 Apr;84(4):549–554. [PMC free article] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Conlan J. W., North R. J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994 Jan 1;179(1):259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Tsang A. Y., Vatter A. E., May M. H. Comparison of 15 laboratory and patient-derived strains of Mycobacterium avium for ability to infect and multiply in cultured human macrophages. J Clin Microbiol. 1986 Nov;24(5):812–821. doi: 10.1128/jcm.24.5.812-821.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F., Maroushek N., Wagner R. D., Steinberg H. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994 Feb 15;152(4):1836–1846. [PubMed] [Google Scholar]
- Denis M., Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993 Nov 15;151(10):5425–5430. [PubMed] [Google Scholar]
- Denis M., Ghadirian E. Interleukin-1 is involved in mouse resistance to Mycobacterium avium. Infect Immun. 1994 Feb;62(2):457–461. doi: 10.1128/iai.62.2.457-461.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991 May 9;324(19):1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- Höglund M., Håkansson L., Venge P. Effects of in vivo administration of G-CSF on neutrophil functions in healthy volunteers. Eur J Haematol. 1997 Mar;58(3):195–202. doi: 10.1111/j.1600-0609.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- Inderlied C. B., Kemper C. A., Bermudez L. E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993 Jul;6(3):266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P., Yeager H., Jr, Whalen G., Evans M., Swartz R. P., Roecklein J. Natural killer cell-mediated lysis of Mycobacterium-avium complex-infected monocytes. J Clin Immunol. 1990 Jan;10(1):71–77. doi: 10.1007/BF00917500. [DOI] [PubMed] [Google Scholar]
- Kayashima S., Tsuru S., Hata N., Rokutanda M. Therapeutic effect of granulocyte colony-stimulating factor (G-CSF) on the protection against Listeria infection in SCID mice. Immunology. 1993 Nov;80(3):471–476. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Yamazaki J., Kasama T., Katsura T., Kasahara K., Wolf S. F., Shimamura T. Interleukin (IL)-12 deficiency in susceptible mice infected with Mycobacterium avium and amelioration of established infection by IL-12 replacement therapy. J Infect Dis. 1996 Sep;174(3):564–573. doi: 10.1093/infdis/174.3.564. [DOI] [PubMed] [Google Scholar]
- Leal I. S., Appelberg R., Silva M. T. Neutrophils are involved in the non-specific resistance to listeriosis induced by mycobacterial infections. Immunology. 1996 Nov;89(3):442–448. doi: 10.1046/j.1365-2567.1996.d01-758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman G. W., Guarnaccia J. R., Remold H. G., Kazanjian P. H., Jr Cytokines enhance neutrophils from human immunodeficiency virus-negative donors and AIDS patients to inhibit the growth of Mycobacterium avium in vitro. J Infect Dis. 1997 Apr;175(4):891–900. doi: 10.1086/513987. [DOI] [PubMed] [Google Scholar]
- Ogata K., Linzer B. A., Zuberi R. I., Ganz T., Lehrer R. I., Catanzaro A. Activity of defensins from human neutrophilic granulocytes against Mycobacterium avium-Mycobacterium intracellulare. Infect Immun. 1992 Nov;60(11):4720–4725. doi: 10.1128/iai.60.11.4720-4725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Immune response to atypical mycobacteria: immunocompetence of heavily infected mice measured in vivo fails to substantiate immunosuppression data obtained in vitro. Infect Immun. 1984 Jan;43(1):32–37. doi: 10.1128/iai.43.1.32-37.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya N., Tsuru S., Katsura Y., Kayashima S., Nomoto K. Enhanced resistance against Listeria monocytogenes achieved by pretreatment with granulocyte colony-stimulating factor. Infect Immun. 1991 Dec;59(12):4740–4743. doi: 10.1128/iai.59.12.4740-4743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Höglund M., Venge P. The effect of granulocyte colony-stimulating factor (G-CSF) on the degranulation of secondary granule proteins from human neutrophils in vivo may be indirect. Br J Haematol. 1996 Jun;93(3):558–568. doi: 10.1046/j.1365-2141.1996.d01-1697.x. [DOI] [PubMed] [Google Scholar]
- Yong K. L. Granulocyte colony-stimulating factor (G-CSF) increases neutrophil migration across vascular endothelium independent of an effect on adhesion: comparison with granulocyte-macrophage colony-stimulating factor (GM-CSF). Br J Haematol. 1996 Jul;94(1):40–47. doi: 10.1046/j.1365-2141.1996.d01-1752.x. [DOI] [PubMed] [Google Scholar]