Abstract
Previous reports have emphasized the requirements for strong type 1 cell-mediated responses in the control of human immunodeficiency virus type 1 (HIV-1). HIV-1 Gag p24-specific CD4 helper T-lymphocyte (HTL) responses have been shown to inversely correlate with viral burden in HIV-1-infected individuals. In this study, peripheral blood mononuclear cells from 70 individuals with chronic progressive HIV-1 infection (clinical progressors), 10 clinical nonprogressors, and 3 immunologically discordant progressors were assessed for HTL proliferation and type 1/type 2 cytokine production. Clinical progressors lacked functional HIV-1-specific HTLs with proliferative and cytokine-producing capacity. Clinical nonprogressors were found to respond to a wide range of HIV-1 antigens from different clades, producing both type 1 and type 2 cytokines. Immunologically discordant progressors responded strongly to clade B Gag p24 with a type 1 cytokine profile but not to other antigens. Thus, in contrast to clinical nonprogressors, neither progressors nor immunologically discordant progressors secreted interleukin-4 (IL-4) in response to HIV-1 antigens. Both clinical nonprogressors and immunologically discordant progressors responded broadly to B clade Gag p24-overlapping peptides. However, IL-4 production in the nonprogressors was restricted to a limited number of p24 peptides. No HIV-1-specific T-cell responses were seen in 20 seronegative controls. Additionally, we observed a rapid type 1 to type 2 shift in the response of one immunologically discordant progressor upon onset of clinical symptoms. These results suggest that a balanced type 1/type 2 profile correlates with successful long-term control of HIV-1.
The apparent failure to maintain or restore human immunodeficiency virus type 1 (HIV-1)-specific memory responses in highly active antiretroviral therapy-treated patients is a major problem and may prevent long-term control of viremia. Untreated clinical nonprogressors control HIV-1 infection over long periods of time, with no diminution in CD4+ T-cell numbers, significantly delaying progression to AIDS. This control of viremia has been attributed to strong HIV-1-specific immune responses (2, 18, 19, 28, 40, 41). Longitudinal studies indicate that a number of apparent clinical nonprogressors show eventual immunological abnormalities and subsequently progress to disease (7, 31).
It has been suggested that genomic mutations and/or deletions found in both the host and the virus could account for the nonprogressor status, but work observing progression to AIDS in patients infected with a nef-deletion strain of HIV-1 stresses the complexity of this issue (14). In contrast to clinical nonprogressors, immunologically discordant progressors manage to control viral replication despite diminution of CD4 T-cell numbers and thus remain an enigma (these patients have also been described as long-term survivors) (38). However, the vast majority of chronically HIV-1-infected individuals fail to control HIV-1 replication, so that high-level viremia results, causing CD4+ T-cell decline despite maintenance of large numbers of HIV-1-specific CD8 T cells (12).
We have shown that an important dysfunction seen in the majority of HIV-1-infected patients from very early in disease is the inability of HIV-1-specific CD4 memory T cells to proliferate and produce interleukin-2 (IL-2) in response to HIV-1 antigens rather than an absolute loss of circulating virus-specific CD4 T cells capable of producing antiviral cytokines (50). Although HIV-1-specific CD8 cytotoxic T lymphocytes (CTL) appear to be essential in limiting viral replication, the mechanisms by which they exert their effector function remain controversial (1, 6, 12, 13). It has, however, become apparent that, as in other viral infections, the presence of functional HIV-1 Gag p24-specific CD4 helper T-lymphocytes (HTL) is crucial in driving the activation of both virus-specific CTL and other HTL as well as in maintaining their effector functions (3, 25, 26, 36, 51).
Thus, CD4 HTL dysfunction in HIV-1 infection, which becomes evident at the earliest stages of the decline in absolute numbers of CD4 T cells and is marked by an early loss of HIV-1-specific responses in terms of both proliferation and type 1 cytokine production (5, 9, 10, 30, 48), might be responsible for a later decline in CTL responses, allowing disease progression (26). Highly active antiretroviral therapy, although able to restore anti-HIV-1 CD4 HTL if given early during primary infection, is unable to rescue these responses in chronically infected individuals (11, 27, 34, 41).
In this study, we performed detailed analyses of HTL responses to a number of HIV-1 antigens and peptides (focusing on p24, the major viral capsid protein, in which multiple highly conserved HTL and CTL epitopes have been defined), in chronically infected clinical progressors, clinical nonprogressors, and immunologically discordant progressors. We assessed the magnitude, breadth, and kinetics of both proliferation and type 1/type 2 cytokine secretion in order to decipher differences in HTL responses which may determine these distinct clinical outcomes. Our data provide evidence that peripheral blood mononuclear cells (PBMC) from clinical nonprogressors exhibit strong and broad responses to many HIV-1 antigens associated with secretion of both type 1 and type 2 cytokines (IL-2 and IL-4, respectively), expressing a normal naïve/memory phenotype. We propose a role for balanced cytokine production in maintaining immune system homeostasis and keeping viral replication in check throughout the course of HIV-1 infection. This most likely represents a complex cross-regulation of the cytokine network, which balances the immune response.
MATERIALS AND METHODS
Study subjects and samples.
Blood samples were taken from 20 uninfected controls and from 10 drug-naïve clinical nonprogressors who had been infected with HIV-1 for more than 5 years and had an undetectable viral load and a stable CD4 T-cell count (median, 824 cells/μl; range, 480 to 1,130 cells/μl). We also studied three immunologically discordant progressors who had been infected with HIV-1 for >10 years, who were defined as showing a decline in CD4 T-cell numbers and yet demonstrating control of their viral load in the absence of antiretroviral treatment (for more detail, see below). In addition, we studied 70 patients with chronic progressive HIV-1 disease (clinical progressors) who were therapy naïve, had a decreasing CD4 T-cell count (median, 285 cells/μl; range, 86 to 420 cells/μl) and high plasma viral load (median, 165,220 copies/ml; range, 5,492 to >500,000 copies/ml). The HLA haplotypes of patients and uninfected donors were assessed by PCR using sequence-specific primers (PCR-SSP) (8). The patients' informed consent and Ethics Committee approval were obtained for the studies described.
Plasma viral RNA assay.
Viral load in patient plasma samples was measured at each time of sample collection with the Bayer HIV-1 RNA 3.0 assay (bDNA) (detection limit, <50 copies/ml; dynamic range, 50 to 500,000 copies/ml; Bayer Diagnostics, Newbury, United Kingdom).
Isolation of PBMC and culture conditions.
PBMC were isolated by density gradient centrifugation and cultured in supplemented RPMI 1640 medium (22, 23). All cell lines were cultured in 10% fetal calf serum-RPMI in 25-cm2 flasks (Greiner Laboritechnik, Dursley, United Kingdom) and incubated at 37°C in 5% CO2.
Antibodies, flow cytometry, and lymphocyte subset quantification.
Murine anti-human CD3, CD4, and CD8 monoclonal antibodies were used (TetraOne; Beckman Coulter, High Wycombe, United Kingdom). The Epics XL-MCL (Beckman Coulter) was used for flow cytometric analyses of total CD3+, CD4+, and CD8+ lymphocytes in whole blood.
Detailed phenotypic evaluation was carried out by three-color flow cytometry on a Becton Dickinson FACScalibur flow cytometer. Quantification of cell surface receptor expression on CD4+ T cells was performed on PBMC samples by multiparameter flow cytometry with commercially available antibodies (Beckman Coulter, High Wycombe, United Kingdom, and PharMingen, Oxford, United Kingdom). Cells were labeled with a cocktail of monoclonal antibodies: anti-CD4 conjugated with phycoerythrin (PE) or with peridinin chlorophyll protein (PerCP); anti-CD45RA-PE or anti-CD45RO-PE; and anti-CD28 conjugated with fluorescein isothiocyanate (FITC), anti-CD62L-FITC, or anti-HLA-DR-FITC for 30 min at 4°C. After staining, cells were washed in phosphate-buffered saline (PBS) and fixed in 2% paraformaldehyde solution in PBS. On acquisition, a gate was set around the lymphocyte population on a forward versus side scatter dot plot, and 10,000 gated events were collected for each sample. Data analysis was performed with CellQuest software, and data are presented as mean percent ± standard error of the mean (SEM). Appropriate isotype-matched negative controls were run in parallel.
Gag p24 peptides and HIV-1 antigens.
Peptides and HIV-1 recombinant antigens were obtained from the Medical Research Council Centralised Facility for AIDS Reagents (National Institute for Biological Standards and Controls, Potters Bar, United Kingdom). The 22 Gag p24 peptides were 20-mers with a 10-amino-acid (aa) overlap covering p24 Gag (aa 133 to 363 of HIV-1 SF2; ARP788.1-22). The HIV-1 recombinant antigens used were described previously (22). Cells were cultured with antigen or peptide at a final concentration of 10 μg/ml. In addition, the inactivated gp120-depleted HIV-1 immunogen (Remune) and its constituent Gag p24 antigen (native p24; clade G) (both from Immune Response Corp., San Diego, Calif.) were used at 3 μg/ml (37).
Proliferation assays.
PBMC (105/well) were cultured with antigen, mitogen, or cytokine in round-bottomed microtiter plates (Greiner) in 10% AB plasma (200 μl; Sigma) in RPMI. The antigens, mitogens, and cytokines used were described previously (22). On day 5, 100 μl of supernatant was collected and stored at −20°C for subsequent cytokine measurement. Each well was then pulsed with 1 μCi of [methyl-3H]thymidine (Amersham International, Amersham, United Kingdom), and 16 h later cells were harvested onto glass fiber filtermats (Wallac Oy, Turku, Finland). Proliferation as measured by [methyl-3H]thymidine incorporation was evaluated by liquid scintillation spectroscopy with a 1205 Betaplate counter (Wallac) (22, 23). Results are expressed as the stimulation index and as the mean counts per minute (cpm) for triplicate cultures, with an error of the mean of <15%. A positive response is defined as a stimulation index value of 5 or more. Control wells for calculation of background activity contained PBMC only.
Bulk cultures.
PBMC at 106/ml were stimulated or not with antigen at the final concentrations described previously in 24-well plates in 14 replicate wells (Greiner). Every 24 h, single cultures were collected and centrifuged at 1,500 rpm for 10 min. Culture supernatants were collected and stored at −20°C until used to measure cytokine production. The remaining cell pellets were immediately used for mRNA isolation as described below.
Measurement of IL-2, IL-4, and IL-10 production.
For cytokine assays, 50 μl of supernatants from proliferative and bulk cultures was transferred to 96-well round-bottomed plates in triplicate for measurement of IL-2, IL-4, and IL-10 on the indicator cell lines CTLL-2 (European Collection of Animal Cell Cultures, Salisbury, United Kingdom), CT.h4S (a generous gift of W. Paul, Bethesda, Md.), and D36 (Deutsche Sammlung von Mikroorganismen, Braunschweig, Germany) as described previously (22, 23, 43). Briefly, CTLL-2 (103 cells/well), CT.h4S (5 × 103 cells/well), or D36 (2 × 103 cells/well in the presence of 16 U of mouse recombinant IL-4 per ml; R&D Sytems, Abingdon, United Kingdom) was added in 50 μl to 50 μl of supernatant to give a final volume of 100 μl. After 24 h in culture, wells were pulsed with [methyl-3H]thymidine, and cells were harvested as described above. Results are expressed as the mean cpm for triplicate cultures, with an error of the mean of <15%. A positive result is defined as significant proliferation above the background activity and detection threshold. In all experiments, a standard titration of indicator cell proliferation to a range of relevant recombinant cytokine from 0.01 to 100 U/ml was included. Control wells for calculation of background activity contained indicator cells only (plus 16 U of mouse recombinant IL-4 per ml for D36 cells).
RNA preparation and first-strand cDNA synthesis.
Stimulated PBMC from HIV-1-infected individuals were obtained at different time points before and after stimulation in bulk cultures, and 106 PBMC were taken for RNA isolation. Total RNA was extracted with a single-step RNA STAT-60 method (AMS Biotechnology, Abingdon, United Kingdom). Samples were then treated with RNase-free DNase I (Boehringer Mannheim, Lewes, United Kingdom) to remove traces of contaminating DNA. The RNA samples were then heated at 65°C for 10 min and placed on ice for a further 10 min, and first-strand cDNA synthesis was obtained with the bulk first-strand cDNA synthesis kit (Pharmacia Biotech, St. Albans, United Kingdom). After 1 h, the reaction was stopped by incubation at 90°C for 5 min, and samples were cooled on ice.
Amplification and detection of cytokine-specific cDNA by reverse transcription-PCR (RT-PCR).
Amplification of gamma interferon (IFN-γ), IL-2, IL-4, and IL-10 gene-specific cDNA was performed as described previously (20). Briefly, primers were for IFN-γ (upstream 5′ primer, 5′GCATCGTTTTGGGTTCTCTTGGCTGTTACTGC3′, and downstream 3′ primer, 5′CTCCTTTTTCGCTTCCCTGTTTTAGCTGCTGG3′), IL-2 (upstream 5′ primer, 5′CATTGCACTAAGTCTTGCACTTGTCA3′, and downstream 3′ primer, 5′CGTTGATATTGCTGATTAAGTCCCTG3′), IL-4 (upstream 5′ primer, 5′CGGCAACTTTGACCACGGACACAAGTGCGATA3′, and downstream 3′ primer, 5′ACGTACTCTGGTTGGCTTCCTTCACAGGACAG3′), and IL-10 (upstream 5′ primer, 5′AAGCTGAGAACCAAGACCCAGACATCAAGGCG3′, and downstream 3′ primer, 5′AGCTATCCCAGAGCCCCAGATCCGATTTTGG3′) (all from Clontech, Basingstoke, United Kingdom), and the standard protocol from Clontech was used. Samples, including appropriate controls (Clontech positive controls; sterile H2O, no cDNA negative controls), were subjected to 35 cycles of amplification of denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 2 min. After amplification, 15 μl of each PCR mixture was resolved on a 1.8% agarose gel (Gibco-BRL Life Technologies, Paisley, United Kingdom), visualized under UV light after ethidium bromide staining, and photographed. A 100-bp DNA ladder (Gibco-BRL) was included to confirm the size of the PCR products.
IFN-γ and IL-4 Elispot assays.
Detection of single-cell IFN-γ and IL-4 release by Elispot was carried out as previously described (22, 29). Briefly, 105 PBMC/well were cultured in 10% AB plasma-RPMI (200 μl; Sigma) in 96-well polyvinylidene difluoride-backed plates (Millipore, Watford, United Kingdom) coated with anti-IFN-γ or anti-IL-4 monoclonal antibodies (Mabtech, Stockholm, Sweden). Cells were stimulated with appropriate peptides at 10 μg/ml or phytohemagglutinin at 10 μg/ml in 10% AB plasma-RPMI (200 μl). Negative controls comprised cells cultured in the presence or absence of an irrelevant peptide. Plates were incubated overnight at 37°C for 16 and for 40 h for detection of IFN-γ and IL-4, respectively. Prior to the development and detection step, supernatants were collected from the IFN-γ and IL-4 plates and stored at −20°C for subsequent measurement of IL-4 and IL-2, respectively, as described above. Spot-forming cells (SFC) were then detected according to the manufacturer's instructions (Mabtech). A positive result is defined as a score of 5 or more SFC above background.
Statistical analysis.
Computer software (Statview 5; Abacus, Berkeley, Calif.) was used for all statistical calculations. Responses to the antigens, mitogens, and cytokines in patients and controls were compared with a Mann-Whitney U test.
RESULTS
HIV-1-specific proliferative responses to HIV-1 antigens in clinical nonprogressors are associated with production of both IL-2 and IL-4.
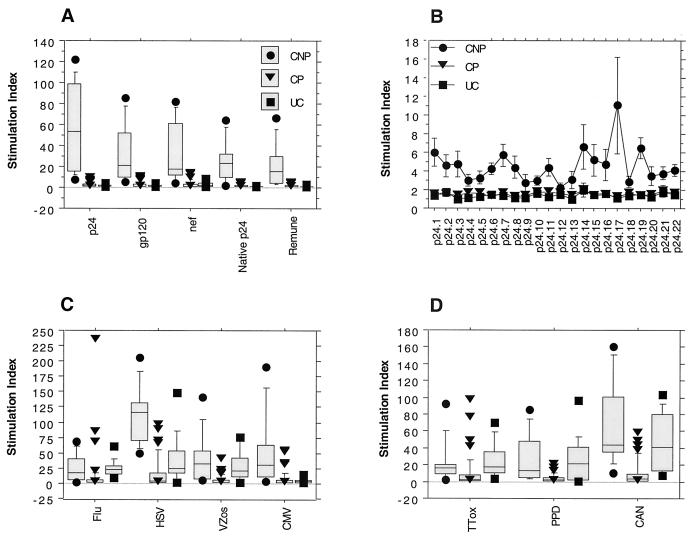
We assessed the magnitude and breadth of both proliferation and cytokine (IL-2 and IL-4) production in response to a wide range of HIV-1 antigens and peptides. All 10 nonprogressors showed strong proliferation in response to all HIV-1 antigens tested (Fig. 1A). Although the most vigorous responses were those directed at recombinant p24 (mean stimulation index ± standard deviation, 60 ± 41), recombinant gp120 and Nef also elicited large responses (stimulation index, 30 ± 28 and 30 ± 29, respectively). In addition, strong responses to whole HIV-1 immunogen (Remune) and native clade G p24 were seen (stimulation index, 26 ± 20 and 25 ± 20, respectively).
FIG. 1.
Lymphocyte proliferation in response to HIV-1 antigens, Gag p24 peptides, and other viral and recall antigens in clinical nonprogressors (CNP), clinical progressors (CP), and uninfected controls (UC). PBMC were cultured in the presence of HIV-1 antigens (A) for 10 clinical nonprogressors, 70 clinical progressors, and 20 uninfected controls; peptides (B) for 10 clinical nonprogressors, 10 clinical progressors, and 10 uninfected controls; and other viral (C) and recall (D) antigens for 10 clinical nonprogressors, 70 clinical progressors, and 20 uninfected controls in triplicate for 6 days, and [3H]thymidine incorporation was measured as described in Materials and Methods. Results are expressed as stimulation indices. Flu, influenza virus; HSV, herpes simplex virus; VZos, varicella-zoster virus; CMV, cytomegalovirus; TTox, tetanus toxoid; PPD, purified protein derivative; CAN, Candida antigen.
Strong anti-p24 responses were accompanied by a wide breadth of responses across overlapping p24 peptides (Fig. 1B, Table 1). Proliferative responses to all HIV-1 antigens tested were absent both in uninfected controls and in 93% of clinical progressors. The responses of nonprogressors to the majority of other viral and recall antigens were comparable to those seen in uninfected controls (Fig. 1C and D). Nonprogressors showed significantly (P < 0.001) increased responses to herpes simplex virus and cytomegalovirus antigens compared to uninfected controls, which concurs with our previous findings (21).
TABLE 1.
Percentage of patients responsive to each peptidea
| Peptide | % of patients
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical nonprogressors (n = 10)
|
Clinical progressors (n = 10)
|
Uninfected controls (n = 10)
|
|||||||
| LPR | IL-2 | IL-4 | LPR | IL-2 | IL-4 | LPR | IL-2 | IL-4 | |
| p24.1 | 50 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.2 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.3 | 40 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.4 | 30 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.5 | 40 | 40 | 20 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.6 | 50 | 50 | 20 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.7 | 60 | 50 | 10 | 10* | 0 | 0 | 10* | 0 | 0 |
| p24.8 | 40 | 30 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.9 | 20 | 20 | 20 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.10 | 30 | 40 | 20 | 10* | 0 | 0 | 0 | 0 | 0 |
| p24.11 | 40 | 50 | 20 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.12 | 10* | 10 | 10 | 10* | 0 | 0 | 10* | 0 | 0 |
| p24.13 | 10 | 30 | 20 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.14 | 50 | 40 | 30 | 10 | 10 | 0 | 10* | 0 | 0 |
| p24.15 | 40 | 30 | 20 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.16 | 20 | 30 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.17 | 50 | 50 | 20 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.18 | 40 | 30 | 20 | 10* | 0 | 0 | 0 | 0 | 0 |
| p24.19 | 70 | 60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.20 | 20 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| p24.21 | 40 | 30 | 10 | 10 | 10 | 0 | 10 | 10 | 0 |
| p24.22 | 30 | 40 | 10 | 10* | 0 | 0 | 0 | 0 | 0 |
LPR, lymphoproliferative response. ∗, borderline positivity (stimulation index = 5). Cutoff for IL-2 assay, 250 cpm; detection threshold, 0.05 U/ml; cutoff for IL-4 assay, 265 cpm; detection threshold, 0.025 U/ml.
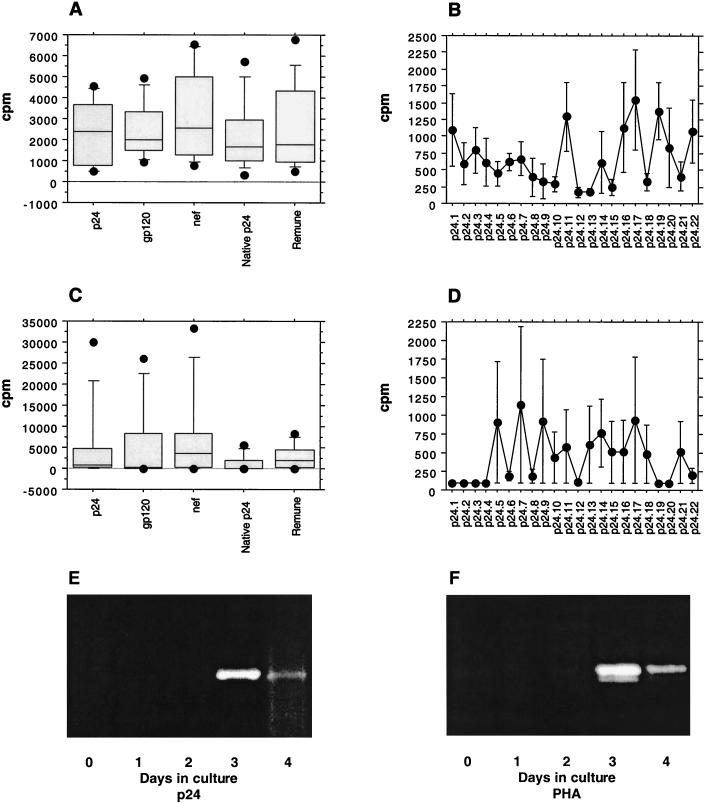
In clinical nonprogressors, IL-2 production was readily detectable in response to all HIV-1 antigens and peptides tested where proliferative responses were seen (Fig. 2A and B, respectively; Table 1). Surprisingly, and in contrast to previous findings, in the majority of nonprogressors (7 of 10), proliferation to all HIV-1 antigens was associated with notable production of IL-4 (Fig. 2C). The p24-induced IL-4 production was due to the ability of the cells to secrete IL-4 in response to a few individual peptides (Fig. 2D; Table 1). Evaluation of the kinetics of p24-induced IL-4-specific mRNA expression assessed during 4 days of culture with RT-PCR revealed that specific IL-4 mRNA expression peaked at day 3 in culture and had declined by day 4 (Fig. 2E). A representative control experiment showing kinetics of IL-4-specific mRNA expression when PBMC were stimulated with phytohemagglutinin is presented in Fig. 2F. The data shown are typical of over five RT-PCRs performed and are consistent with the bioassay results.
FIG. 2.
IL-2 and IL-4 production in response to HIV-1 antigens and Gag p24 peptides in 10 clinical nonprogressors. PBMC were cultured in the presence of HIV-1 antigens or peptides in triplicate for 5 days, and IL-2 and IL-4 production in response to HIV-1 antigens (A and C, respectively) or peptides (B and D, respectively) was measured in culture supernatant by proliferation of the indicator cell lines CTLL-2 and CT.h4S. Results are expressed as the mean cpm for triplicate cultures with an error of the mean of <15%. (E and F) Representative experiments showing kinetics of IL-4 mRNA expression after stimulation of clinical nonprogressor PBMC with recombinant p24 (E) or phytohemagglutinin (F) over a 4-day culture (RT-PCR products of IL-4 mRNA expression on days 0 to 4 in culture).
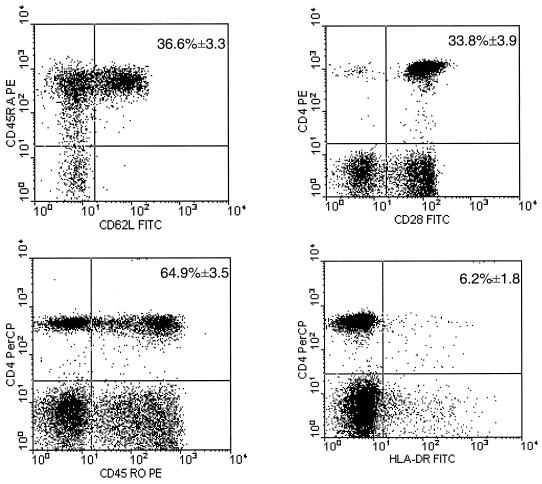
Phenotypic analysis of peripheral blood CD4+ T cells from clinical nonprogressors revealed normal percentages of subpopulations of naïve (CD4CD45RA) and memory/effector (CD4CD45RO) CD4+ T cells (mean ± SEM, 36.6% ± 3.3% and 64.9% ± 3.5%, respectively) comparable to those seen in uninfected or seronegative controls (17) (Fig. 3). The majority of CD4+ T cells expressed the costimulatory molecule CD28 (32) (mean ± SEM = 33.8% ± 3.9%), while the majority of CD4+ T cells did not express HLA-DR (mean ± SEM = 6.2% ± 1.8%), suggesting that these were resting CD4+ T cells (17) (Fig. 3).
FIG. 3.
Phenotypic analysis of peripheral blood CD4+ T cells in clinical nonprogressors. A representative experiment showing the percentage of CD45RA+ CD62L+ (true naïve), CD45RO+ (effector/memory), CD28+ (costimulatory), and HLA-DR+ (activated) CD4+ T lymphocytes in clinical nonprogressors. The means ± SEM for all HIV-1-positive clinical nonprogressors are shown in the upper right quadrant. Phenotypic analysis of CD4 T-cell subsets was carried out by multiparameter flow cytometry as described in Materials and Methods.
Presence or absence of IL-4 does not affect strength or breadth of HIV-1-specific responses in clinical nonprogressors.
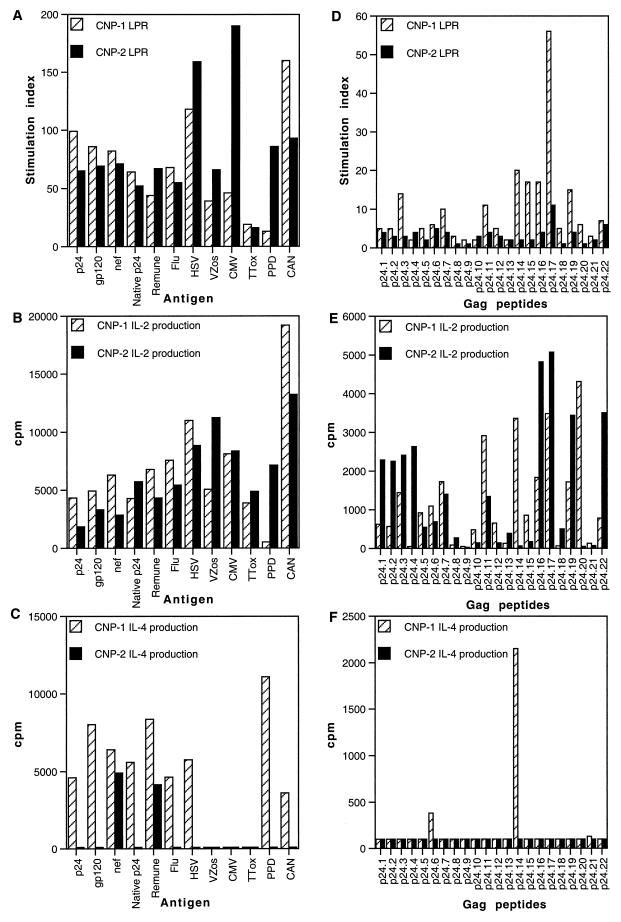
Two drug-naïve nonprogressor patients who had stable or increasing CD4 T-cell counts (480 and 1,052 cells/μl) and undetectable viral loads (<50 copies/ml) and had been infected with HIV-1 for >8 years were selected from this cohort based on in vitro CD4 HTL responses and studied in more detail. Both individuals showed comparable proliferative responses and broad IL-2-producing capacity in response to all antigens and peptides tested (Fig. 4A, B, D, and E). Strong p24-, gp120-, and Nef-specific responses (mean stimulation index = 79; stimulation index range, 65 to 99) as well as strong responses to whole HIV-1 immunogen and native clade G p24 (mean stimulation index = 57; stimulation index range, 44 to 67) were observed in both clinical nonprogressors.
FIG. 4.
Lymphoproliferative responses (LPR) and IL-2 and IL-4 production in response to HIV-1 antigens, other viral and recall antigens, and Gag p24 peptides in clinical nonprogressor patients 1 and 2 (CNP-1 and CNP-2). PBMC from two clinical nonprogressor patients were cultured in the presence of various antigens (A) or peptides (D) in triplicate for 6 days, and [3H]thymidine incorporation was measured as described in Materials and Methods. Results are expressed as stimulation indices. IL-2 production in response to various antigens (B) or peptides (E) was measured in culture supernatants by proliferation of the indicator cell line CTLL-2, and IL-4 production in response to various antigens (C) or peptides (F) was measured in culture supernatants by proliferation of the indicator cell line CT.h4S. Results are expressed as the mean cpm for triplicate cultures, with an error of the mean of <15%. See Fig. 1 legend for other abbreviations.
The responses of nonprogressors to recall antigens, mitogens, and IL-2 were comparable to those seen in uninfected controls. For recall antigens, the mean stimulation index was 81, and the stimulation index range was 13 to 190 (Fig. 4A), and, as previously described, for mitogens the mean stimulation index was 319 and the stimulation index range was 55 to 559 and for IL-2 the mean stimulation index was 335 and the stimulation index range was 305 to 373 (21). However, IL-4-producing capacity was limited, and differential IL-4-inducing capacity in response to recombinant p24, clade G p24, and overlapping Gag peptides as well as gp120, influenza virus, herpes simplex virus, purified protein derivative, and Candida antigens was observed (Fig. 4C and F). In PBMC from clinical nonprogressor patient 1, Gag p24 induced significant IL-4 production mainly in response to peptides 14 and 6, whereas in clinical nonprogressor patient 2 notable amounts of IL-4 were observed only in response to recombinant Nef and Remune, while no IL-4 was produced in response to either recombinant p24, clade G p24, or Gag peptides (Fig. 4C and F). This response to Remune is likely to be directed at HIV-1 antigens other than p24 contained in the immunogen (R. Moss, personal communication).
HIV-1-specific responses in immunologically discordant progressors are mainly restricted to p24.
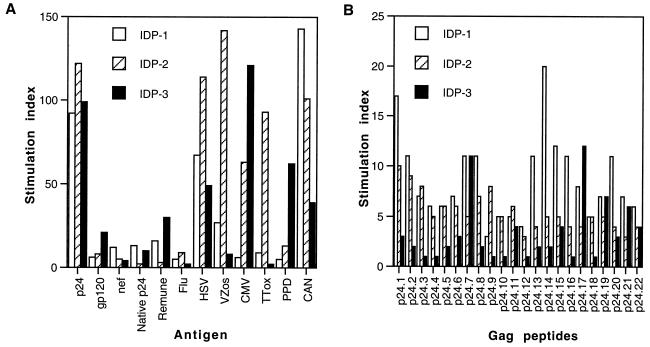
Three drug-naïve immunologically discordant progressor patients were studied who, despite declining CD4+ T-cell counts (240, 450, and 346 cells/μl), maintained control of HIV-1 replication (for two patients, <50 copies/ml, and for patient 1, with the lowest CD4+ T-cell count, 1,236 copies/ml). Strong proliferative responses to HIV-1 Gag p24 recombinant antigen were seen in all three patients (mean stimulation index = 104; stimulation index range, 92 to 122; Fig. 5A). However, gp120- and Nef-specific responses (although significant) were lower than those to p24 (mean stimulation index = 9.5; stimulation index range, 4 to 21), in contrast to the results obtained with the clinical nonprogressors. Two of the three patients responded to both native p24 and Remune (mean stimulation index = 17; stimulation index range, 10 to 30; Fig. 5A).
FIG. 5.
Lymphocyte proliferation in response to HIV-1 antigens, other viral and recall antigens, and Gag p24 peptides in immunologically discordant progressors. PBMC from immunologically discordant progressor patients 1, 2, and 3 (IDP-1, IDP-2, and IDP-3, respectively) were cultured in the presence of various antigens (A) or peptides (B) in triplicate for 6 days, and [3H]thymidine incorporation was measured as described in Materials and Methods. Results are expressed as stimulation indices. See Fig. 1 legend for other abbreviations.
Strong p24-specific lymphoproliferative responses were reflected in the breadth of the responses to p24 peptides (Fig. 5B). Proliferation was in all cases associated with IL-2 production, while no IL-4 was detected in cultures (data not shown). The responses of immunologically discordant progressors to recall antigens, mitogens, and IL-2 were lower than those seen in nonprogressor patients and uninfected controls. For recall antigens, the mean stimulation index was 51 and the stimulation index range was 2 to 143 (Fig. 5A); for mitogens, the mean stimulation index was 80 and the stimulation index range was 21 to 142; and for IL-2, the mean stimulation index was 189 and the stimulation index range was 116 to 235 (data not shown).
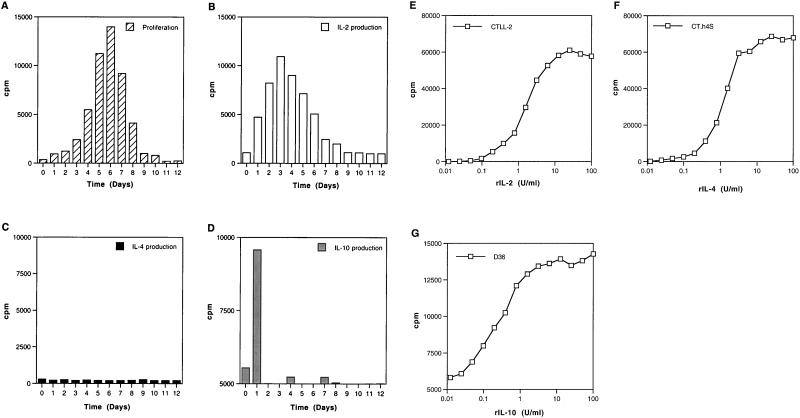
Kinetics of p24-specific responses in immunologically discordant progressor 2: proliferation and cytokine profiles.
Since immunologically discordant progressor patient 2 showed the strongest anti-p24 responses in the immunologically discordant progressor group, we chose to investigate these responses in more detail. The kinetics of p24-specific proliferative responses were consistent and revealed maximum proliferation between days 5 and 6, followed by a gradual decline (Fig. 6A). Aliquots of culture supernatants were collected from bulk cultures at different time points to assess the time course of cytokine production following activation with recombinant p24. Production of IL-2 was found to peak at day 3 in culture, after which it declined gradually and disappeared after day 7 (Fig. 6B). Lack of IL-4 detection (Fig. 6C) did not reflect its consumption or utilization, since IL-4 was not detected throughout the 12 days of culture, which concurs with the RT-PCR analysis (see below). Secretion of IL-10 was found to peak within the first 24 h of stimulation, at day 1 of culture, followed by a dramatic fall (Fig. 6D).
FIG. 6.
Kinetics of lymphocyte proliferation and cytokine production in response to recombinant p24 antigen in immunologically discordant progressor patient 2. (A) Kinetics of proliferation in response to recombinant p24 over a 12-day culture. PBMC from immunologically discordant progressor patient 2 were cultured in the presence of p24, and cultures were pulsed and harvested at 24-h intervals. Results are expressed as mean cpm for triplicate cultures, with an error of the mean of <15%. Kinetics of IL-2 (B), IL-4 (C), and IL-10 (D) production in response to recombinant p24 were measured in culture supernatants collected at 24-h intervals as described in Materials and Methods, by proliferation of the indicator cell lines CTLL-2, CT.h4S, and D36, respectively. All three cell lines respond in a dose-dependent manner, and a standard titration was included in each experiment (E, F, and G, respectively). Results are expressed as the mean cpm for triplicate cultures, with an error of the mean of <15%.
In order to demonstrate the sensitivity of our bioassays, the responses of these cells (CTLL-2, CT.h4S, and D36) to a range of concentrations of recombinant IL-2 (rIL-2), rIL-4, and rIL-10, respectively, were included in each experiment (Fig. 6E to G). These cells consistently proliferated to human recombinant cytokines in a dose-dependent manner, with significant proliferation above background control levels occurring for rIL-2 at a concentration of 0.05 U/ml with CTLL-2 cells, for rIL-4 at a concentration of 0.025 U/ml with CT.h4S cells, and for rIL-10 at a concentration of 0.01 U/ml with D36 cells. Bioassays are in general more sensitive than enzyme-linked immunosorbent assay (ELISA) (23, 43), and others have shown that similar levels and kinetics are obtained when supernatants are assessed in parallel with bioassays and ELISA (24). However, the best correlations are obtained when bioassays are compared with RT-PCR analysis (Fig. 2C, 2E, 6, and 7).
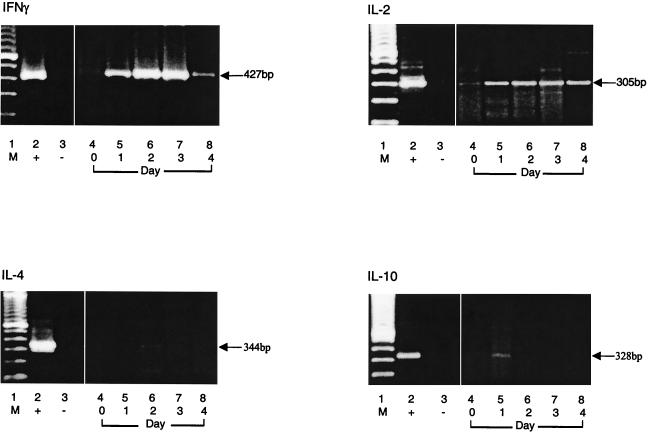
RT-PCR analysis of cytokine-specific mRNA in immunologically discordant progressor 2.
RT-PCR analysis was used to confirm changes in expression of type 1 and type 2 cytokine-specific mRNAs occurring during 4 days of culture (Fig. 7). Specific IFN-γ, IL-2, IL-4, and IL-10 mRNA expression was determined by RT-PCR, which showed strong expression of specific IFN-γ mRNA after 24 h in culture that increased dramatically on days 2 and 3 and then diminished by day 4. IL-2 mRNA expression, although at lower levels, occurred early in culture (day 1) and persisted throughout. In contrast to the clinical nonprogressors, in whom IL-4 was detected at days 3 to 4 in culture, IL-4-specific mRNA remained undetectable throughout p24 stimulation for the immunologically discordant progressors. IL-10-specific mRNA expression was visible at 24 h, after which it disappeared. Thus, RT-PCR analysis concurs with the data obtained in our bioassays.
FIG. 7.
Gel electrophoresis of RT-PCR products of IFN-γ, IL-2, IL-4, and IL-10 mRNAs obtained after stimulation with recombinant p24. PBMC from immunologically discordant progressor patient 2 harvested from the bulk cultures were subjected to mRNA isolation and RT-PCR as described in Materials and Methods. Lane 1, DNA size markers; lane 2, positive control; lane 3, negative control; lane 4, day 0; lanes 5 to 8, days 1 to 4 of stimulation with recombinant p24, respectively.
During clinical progression, both CD4+ and CD8+ HIV-1-specific T-cell responses are associated with increased IL-4 production, decreased IFN-γ production, and absence of IL-2.
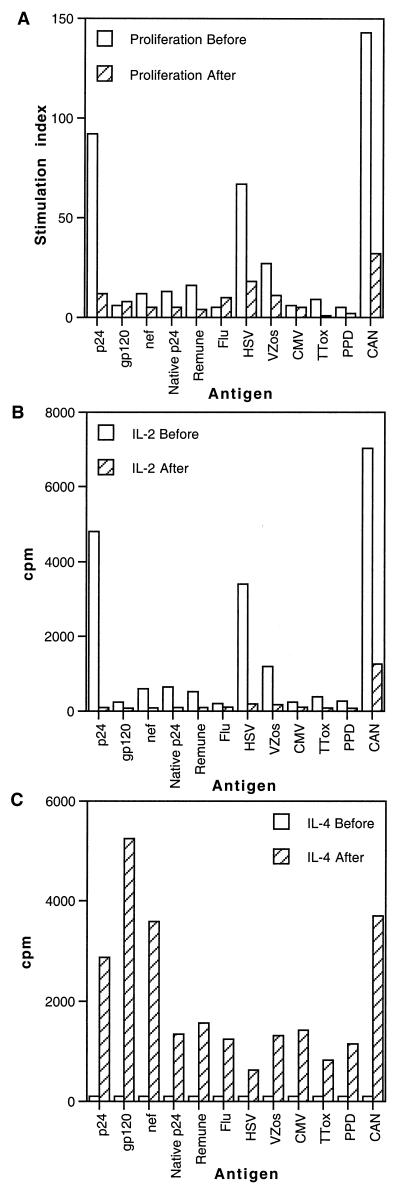
Follow-up of immunologically discordant progressor patient 1 revealed that clinical progression to symptomatic disease was associated with a drop in CD4 T-cell count from 240 to 201 cells/μl and an increase in viral load from 1,236 to 6,483 copies/ml in 6 months. Although these changes in CD4 T-cell number and viral load were moderate, they were associated with a decline in both proliferation and IL-2 production in response to all antigens tested (Fig. 8A and B). However, a dramatic increase in the secretion of IL-4 in response to all the antigens tested was apparent (Fig. 8C).
FIG. 8.
Lymphocyte proliferation and IL-2 and IL-4 production in response to HIV-1 antigens and other viral and recall antigens in immunologically discordant progressor patient 1 before and after progression. (A) PBMC from immunologically discordant progressor patient 1 obtained before and after progression were cultured in the presence of various antigens in triplicate for 6 days, and [3H]thymidine incorporation was measured as described in Materials and Methods. Results are expressed as stimulation indices. IL-2 (B) and IL-4 (C) production in response to various antigens was measured in culture supernatants by proliferation of the indicator cell lines CTLL-2, and CT.h4S, respectively. Results are expressed as the mean cpm for triplicate cultures, with an error of the mean of <15%. See Fig. 1 legend for abbreviations.
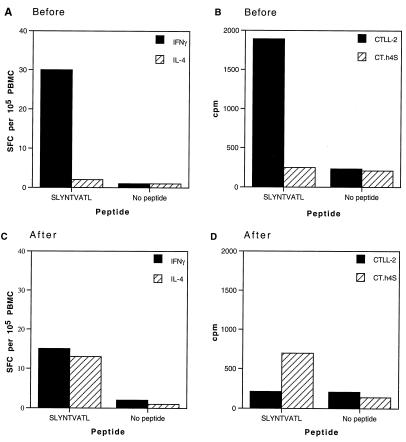
In order to establish whether this IL-4-secreting capacity extended to CD8 T cells, we carried out both IFN-γ and IL-4 Elispot assays, in which cells were stimulated with appropriate major histocompatibility complex (MHC) class I-restricted peptides. Before progression, activation with relevant peptide induced significant levels of IFN-γ SFC, accompanied by secretion of substantial amounts of IL-2 in culture (Fig. 9A and B). However, upon progression to disease, there was a decrease in IFN-γ SFC, an absence of IL-2 in culture, and an increase in IL-4 SFC, which was confirmed by measurable levels of IL-4 in culture supernatants with the CT.h4S bioassay (Fig. 9C and D). Additional follow-up analysis on this patient at two subsequent time points 6 months apart showed that lack of overall responses remained a reproducible finding and was associated with low CD4 count and a persistent viral load of ∼10,000 copies/ml, confirming that this patient had indeed progressed.
FIG. 9.
Type 1 and type 2 cytokine production by CD8+ T cells in response to HLA-A*0201-restricted peptide in immunologically discordant progressor patient 1 before and after clinical progression. Elispot assays measuring IFN-γ and IL-4 release in response to HLA-A*0201-restricted Gag peptide (SLYNTVATL) before (A) and after (C) progression. PBMC were cultured in the presence or absence of relevant peptide, and Elispot assays were carried out as described in Materials and Methods. Data represent mean values at each point, and variation among duplicates was <20%. Culture supernatants were collected for subsequent measurement of IL-4 and IL-2 with bioassays. IL-2 and IL-4 release in response to SLYNTVATL peptide before (B) and after (D) progression was measured in culture supernatants by proliferation of the indicator cell lines CTLL-2 and CT.h4S, respectively. The HLA phenotype of immunologically discordant progressor patient 1 was A0201/2501, B1801/62, Bw6, C10/1203, DRB1 1501, DQB1 8.
DISCUSSION
Design of successful immunotherapeutic interventions for chronic HIV-1 infection during highly active antiretroviral therapy is dependent on our understanding of HIV-1-specific immune responses observed in clinical nonprogressors. Such interventions should guarantee protection from HIV-1 disease by controlling viremia and preventing the onset of AIDS. In this study, we examined the magnitude, breadth, and kinetics of the CD4 T-cell component of the anti-HIV-1 immune responses in patients with undetectable or low viral loads who maintained strong proliferative responses, particularly to Gag p24. We present data concerning the quantity and quality of anti-HIV-1 T-cell responses observed in clinical nonprogressors and immunologically discordant progressors that lead to effective control of viremia.
Although the effective suppression of viral replication seen in nonprogressors has been previously primarily attributed to anti-HIV-1 CD8 T cells, it is apparent that, as in other viral infections, regulatory CD4 HTL play a pivotal role in the maintenance of CD8 T-cell responses (3, 13, 25, 26, 36, 41, 51). HIV-1-specific CD8 T cells that lack effector function have been reported in chronically HIV-1-infected individuals (1, 12). Our previous studies show similar findings for HIV-1-specific CD4 T cells, where loss of proliferation and IL-2 production rather than loss of circulating virus-specific T cells capable of producing the antiviral cytokines tumor necrosis factor alpha and IFN-γ was observed in chronic progressors (50). Indeed, it is the proliferative dysfunction of CD4 T cells seen early during infection and before any decline in CD4 T-cell numbers that has been suggested to be responsible for their inability to support virus-specific CD8 T-cell effector function (26).
Although the strong anti-p24 responses found in nonprogressors have been linked to control of viremia (41), our data show that this is also true in the immunologically discordant progressors. In contrast to previous reports, clinical nonprogressors, who were characterized by normal and stable CD4 T-cell counts and undetectable viral loads, displayed robust anti-HIV-1 responses to all HIV-1 antigens tested (including clade B p24, gp120, and Nef as well as clade G p24 and the whole HIV-1 immunogen Remune), showing CD4+ T-cell cross-reactivity to both clade B and G. Anti-p24 responses specific for the majority of Gag peptides were present, indicating that perhaps MHC class II restriction is more promiscuous than class I.
Proliferation was always accompanied by significant production of IL-2. Furthermore, substantial secretion of IL-4 was detected in cultures of 7 of 10 clinical nonprogressors and across the majority of HIV-1 antigens and peptides tested. Immune responses to other recall antigens, mitogens, and IL-2 were comparable to those observed in HIV-1-seronegative controls. The increased responses to herpes simplex virus and cytomegalovirus antigens most likely reflect the higher prevalence of these infections within the HIV-1-infected population. In a representative patient, clinical nonprogressor 1, IL-4 production was seen in response to all antigens tested except varicella-zoster virus, cytomegalovirus, and tetanus toxoid.
While IL-4 was produced in response to all p24 antigens tested, when individual p24 peptides were assessed for the ability to induce secretion of IL-4, it was revealed that this IL-4-inducing capacity was restricted to peptides 14 and 6. The strength of p24-specific responses was not affected by the differential ability of PBMC to release IL-4 in response to p24 antigen and/or peptides, since in this case the presence of IL-4 in the culture did not have a detrimental effect on either proliferation or the IL-2-producing capacity of the anti-HIV-1 HTL response. This was also the case for clinical nonprogressor patient 2, in whom IL-4 production was seen only in response to Nef and Remune.
These data might reflect cross-modulation and cross-regulation between type 1 and type 2 cytokines (particularly their characteristic products, IFN-γ and IL-4, known to often oppose each other's action), which may be crucial in maintaining a consistent cellular response in HIV-1-infected nonprogressors, or may reflect the intrinsic ability of individuals to produce type 2 cytokines due to a previous anamnestic encounter with other pathogens (44, 46). Alternatively, restricted numbers of p24 peptides might act as altered peptide ligands for particular CD4 T-cell subsets or clones, resulting in a qualitatively different pattern of signaling and subsequently in different functional capacity (45). Such a phenomenon may well be responsible for shaping the T-cell-mediated immune responses to HIV-1.
Immunologically discordant progressors, characterized by decreasing CD4 T-cell counts and undetectable or low viral loads, displayed equally vigorous anti-HIV-1 responses, which were directed mainly towards recombinant clade B p24. These anti-p24 responses were approximately 10-fold higher than those seen towards the other HIV-1 antigens tested, and the strength of p24 antigen-specific response was reflected in equally broad responses to the panel of overlapping p24 peptides. Responses to other recall antigens, mitogens, and IL-2 were approximately twofold lower than those seen for clinical nonprogressors and seronegative controls. IL-2 but not IL-4 was readily detected in cultures where significant proliferation was seen. This concurs with previous findings and with the rationale of successful prophylactic vaccination, which is aimed at developing strong type 1 cell-mediated responses, which should not be affected by type 2 responses (39, 41).
Our findings raise two important questions. First, are the anti-p24 responses seen in immunologically discordant progressors protective? It is possible that the p24-specific responses are of primary importance and that clinical nonprogressors make good responses to other antigens only because those responses remain intact, as they have not been depleted by disease progression. The second important question is why do CD4 T-cell numbers decline in immunologically discordant progressors despite controlled viremia? The mechanism behind this phenomenon is difficult to explain and may involve increased sensitivity to one or more factors causative of indirect virus-mediated cytopathic effects, such as activation-induced cell death or multinucleated cell syncytium formation (47). Clonal deletion and/or anergy may result from a variety of indirect mechanisms of HIV-1 infection. These include hyperactivation, activation-induced cell death of uninfected T cells, and damage or destruction of regenerative compartments (thymus and/or bone marrow) (15). Different levels of genetic susceptibility of the host to these mechanisms may explain CD4 T-cell depletion in the presence of low viremia.
The kinetics of p24-specific response in an immunologically discordant progressor reflected a predominant presence of type 1 cytokine responses, with the immunosuppressive type 2 cytokine IL-10 seen only transiently at the beginning of culture. A strong type 1 cytokine (IFN-γ and IL-2) profile in response to p24 suggests suppression of virus replication; however, in addition, it could also suggest that these anti-p24 responses are inappropriately skewed. Such a polarized immune response may be indicative of a hyperactivated state. However, our data do not support this hypothesis, as the breadth of response to the Gag p24 peptides was comparable in immunologically discordant progressors and in nonprogressors.
A very strong type 1 response might be detrimental in some HIV-1-infected patients in whom viremia is controlled but the indirect bystander effects of the immune activation (possibly of a type 1) may be important. This would concur with work by Zinkernagel et al. and implicates anti-HIV-1 T-cell responses in immune-mediated pathology (52). We suggest that a balanced type 1/type 2 T-cell profile is indicative of a nonpolarized immune response which has not been skewed by hyperactivation. In addition, the kinetics of cytokine production at both the protein and mRNA level suggest strong type 1 p24-induced responses, with only transient detection of the immunosuppressive cytokine IL-10. A sharp but very transient peak of IL-10 production at day 1 is likely to be due to nonspecific or innate immune cells such as monocytes and may not represent type 2 polarization.
Our data suggest that in individuals who control viremia through maintenance of p24-specific responses, there is a minimal requirement for cross-regulation of immune responses or these type 1 responses are cross-regulated efficiently by brief transient type 2 cytokine (IL-10) production. The data may indicate reduced immune activation in immunologically discordant progressors compared with that observed in chronic progressive HIV-1 infection, with no need for the IL-10-secreting anergic suppressor T-cell subset (33, 49). Commitment to IFN-γ or IL-4 production does not necessarily require proliferation and cell division (4), and thus, loss of a particular virus-specific cytokine-secreting subset might simply reflect preferential infection with HIV-1 (42).
Clinical progression in one immunologically discordant progressor patient, although accompanied by only moderate changes in CD4 T-cell count and viral load, resulted in dramatic immunological changes and was associated with a diminution of type 1 responses and a substantial release of the type 2 cytokine IL-4 by HIV-1-specific CD4 and CD8 T cells. Reduced proliferation in response to all antigens and peptides tested was associated with an absence of IL-2 production except for the Candida antigen, with which proliferation was minimal. This absence of IL-2 production was accompanied by increased production of IL-4 in response to all antigens tested and was maximal for all three HIV-1 antigens tested (p24, gp120, and Nef) and Candida antigen. This increased production of IL-4 also extended to HIV-1 peptide-specific CD8 T cells and was accompanied by an overall reduction in expression of CD28 (data not shown), which is known to be downmodulated by IL-4 and which correlates with virus replication and dissemination (16, 35).
Our findings confirm that HIV-1-specific CD4 T cells are present but have been rendered anergic directly by HIV-1 or indirectly by clonal suppression. The dramatic increase in IL-4 secretion suggests that cells are responsive to antigenic challenge, but because of the immunosuppressive microenvironment, the cells are only capable of responding with secretion of IL-4. Induction of type 2 immunity may not necessarily diminish the quantity and quality of cell-mediated immune responses required to be elicited when a vaccine antigen is administered, but such data must be taken into consideration when designing prime-boost strategies (39).
Conclusions regarding correlations between the secretion of immunoregulatory cytokines such as IL-4 and IL-10 and disease progression should be considered carefully, since although IL-4 secretion was enhanced with disease progression in one immunologically discordant progressor, in the clinical nonprogressor patients secretion of IL-4 did not affect virus-specific T-cell responses. Our data raise questions for prophylactic approaches where the rationale may be to induce a protective immune response that controls HIV-1 but does not entirely eliminate HIV-1 and also for therapeutic approaches where the aim is to induce and maintain protective responses in an immunosuppressed microenvironment.
Acknowledgments
We thank the patients and staff at Chelsea & Westminster Hospital, St. George's Hospital, and the Royal Free Hospital who participated in this study; Ron Moss from the Immune Response Corp. for the whole HIV-1 immunogen and the native clade G p24; and K. Begolli for computing assistance.
The reagents ARP788.1-22, EVA 620, EVA 646, and EVA 650 were provided by the EU Programme EVA/MRC Centralised Facility for AIDS Reagents, NIBSC, United Kingdom (grants QLK2-CT-1999-00609 and GP828102). This work was supported by the Wellcome Trust (grant 058700).
REFERENCES
- 1.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. A. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. L. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker, E., C. E. Mackewicz, G. Reyes-Teran, A. Sato, S. A. Stranford, S. H. Fujimura, C. Christopherson, S. Y. Chang, and J. A. Levy. 1998. Virological and immunological features of long-term human immunodeficiency virus-infected individuals who have remained asymptomatic compared with those who have progressed to acquired immunodeficiency syndrome. Blood 92:3105-3114. [PubMed] [Google Scholar]
- 3.Battegay, M., D. Moskophidis, A. Rahemtulla, H. Hengartner, T. W. Mak, and R. M. Zinkernagel. 1994. Enhanced establishment of a virus carrier state in adult CD4+-T-cell-deficient mice. J. Virol. 68:4700-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Sasson, S. Z., R. Gerstel, J. Hu-Li, and W. E. Paul. 2001. Cell division is not a “clock” measuring acquisition of competence to produce IFN-gamma or IL-4. J. Immunol. 166:112-120. [DOI] [PubMed] [Google Scholar]
- 5.Berzofsky, J. A., A. Bensussan, K. B. Cease, J. F. Bourge, R. Cheynier, Z. Lurhuma, J. J. Salaun, R. C. Gallo, G. M. Shearer, and D. Zagury. 1988. Antigenic peptides recognized by T lymphocytes from AIDS viral envelope-immune humans. Nature 334:706-708. [DOI] [PubMed] [Google Scholar]
- 6.Brodie, S. J., D. A. Lewinsohn, B. K. Patterson, D. Jiyamapa, J. Krieger, L. Corey, P. D. Greenberg, and S. R. Riddell. 1999. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat. Med. 5:34-41. [DOI] [PubMed] [Google Scholar]
- 7.Buchbinder, S. P., M. H. Katz, N. A. Hessol, P. M. O'Malley, and S. D. Holmberg. 1994. Long-term HIV-1 infection without immunologic progression. AIDS 8:1123-1128. [DOI] [PubMed] [Google Scholar]
- 8.Bunce, M., C. M. O'Neill, M. C. N. M. Barnardo, P. Karusa, M. J. Browning, P. J. Morris, and K. I. Welsh. 1995. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB3, DRB4, DRB5 and DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP). Tissue Antigens 46:355-367. [DOI] [PubMed] [Google Scholar]
- 9.Clerici, M., N. I. Stocks, R. A. Zajac, R. N. Boswell, D. C. Bernstein, D. L. Mann, G. M. Shearer, and J. A. Berzofsky. 1989. Interleukin-2 production used to detect antigenic peptide recognition by T-helper lymphocytes from asymptomatic HIV-seropositive individuals. Nature 339:383-385. [DOI] [PubMed] [Google Scholar]
- 10.Clerici, M., N. I. Stocks, R. A. Zajac, R. N. Boswell, D. R. Lucey, C. S. Via, and G. M. Shearer. 1989. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J. Clin. Investig. 84:1892-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connors, M., J. A. Kovacs, S. Krevat, J. C. Gea-Banacloche, M. C. Sneller, M. Flanigan, J. A. Metcalf, R. E. Walker, J. Falloon, M. Baseler, I. Feuerstein, H. Masur, and H. C. Lane. 1997. HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiviral or immune-based therapies. Nat. Med. 3:533-540. [DOI] [PubMed] [Google Scholar]
- 12.Gea-Banacloche, J. C., S. A. Migueles, L. Martino, W. L. Shupert, A. C. McNiel, M. S. Sabbaghian, L. Ehler, C. Prussin, R. Stevens, L. Lambert, J. Altman, C. W. Hallahan, J. C. de Quiros, and M. Connors. 2000. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J. Immunol. 165:1082-1092. [DOI] [PubMed] [Google Scholar]
- 13.Goulder, P. J. R., S. L. Rowland-Jones, A. J. McMichael, and B. D. Walker. 1999. Anti-HIV cellular immunity: recent advances towards vaccine design. AIDS 13(Suppl. A):S121-S136. [PubMed] [Google Scholar]
- 14.Greenough, T. C., J. L. Sullivan, and R. C. Desrosiers. 1999. Declining CD4 T-cell counts in a person infected with Nef-deleted HIV-1. N. Engl. J. Med. 340:236-237. [DOI] [PubMed] [Google Scholar]
- 15.Grossman, Z., M. Meier-Schellersheim, A. E. Sousa, R. M. Victorino, and W. E. Paul. 2002. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 8:319-323. [DOI] [PubMed] [Google Scholar]
- 16.Haffar, O. K., M. D. Smithgall, J. G. Wong, J. Bradshaw, and P. S. Linsley. 1995. Human immunodeficiency virus type 1 infection of CD4+ T cells down-regulates the expression of CD28: effect on T-cell activation and cytokine production. Clin. Immunol. Immunopathol. 77:262-270. [DOI] [PubMed] [Google Scholar]
- 17.Hannet, I., F. Erkeller-Yuksel, P. Lydyard, V. Deneys, and M. DeBruyere. 1992. Developmental and maturational changes in human blood lymphocyte subpopulations. Immunol. Today 13:215-218. [DOI] [PubMed] [Google Scholar]
- 18.Harrer, T., E. Harrer, S. A. Kalams, P. Barbosa, A. Trocha, R. P. Johnson, T. Elbeik, M. B. Feinberg, S. P. Buchbinder, and B. D. Walker. 1996. Cytotoxic T lymphocytes in asymptomatic long-term nonprogressing HIV-1 infection. Breadth and specificity of the response and relation to in vivo viral quasispecies in a person with prolonged infection and low viral load. J. Immunol. 156:2616-2623. [PubMed] [Google Scholar]
- 19.Harrer, T., E. Harrer, S. A. Kalams, T. Elbeik, S. I. Staprans, M. B. Feinberg, Y. Cao, D. D. Ho, T. Yilma, A. M. Caliendo, R. P. Johnson, S. P. Buchbinder, and B. D. Walker. 1996. Strong cytotoxic T-cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res. Hum. Retrovir. 12:585-592. [DOI] [PubMed] [Google Scholar]
- 20.Imami, N., C. Antonopoulos, G. A. D. Hardy, B. Gazzard, and F. M. Gotch. 1999. Assessment of type 1 and type 2 cytokines in HIV-1-infected individuals: impact of highly active antiretroviral therapy. AIDS Res. Hum. Retrovir. 15:1499-1508. [DOI] [PubMed] [Google Scholar]
- 21.Imami, N., G. Hardy, C. Burton, A. Pires, J. Pido-Lopez, R. Moss, B. Gazzard, and F. Gotch. 2001. Immune responses and reconstitution in HIV-1-infected individuals: impact of anti-retroviral therapy, cytokines and therapeutic vaccination. Immunol. Lett. 79:63-76. [DOI] [PubMed] [Google Scholar]
- 22.Imami, N., G. A. D. Hardy, M. R. Nelson, S. Morris-Jones, R. Al-Shahi, C. Antonopoulos, B. Gazzard, and F. M. Gotch. 1999. Induction of HIV-1-specific T-cell responses by administration of cytokines in late-stage patients receiving highly active antiretroviral therapy. Clin. Exp. Immunol. 118:78-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imami, N., M. Larché, and M. A. Ritter. 1994. Inhibition of alloreactivity by monoclonal antibodies MR6: differential effects on IL-2- and IL-4-producing human T cells. Int. Immunol. 6:1575-1584. [DOI] [PubMed] [Google Scholar]
- 24.Jordan, W. J., and M. A. Ritter. 2002. Optimal analysis of composite cytokine responses during alloreactivity. J. Immunol. Methods 260:1-14. [DOI] [PubMed] [Google Scholar]
- 25.Kalams, S. A., S. P. Buchbinder, E. S. Rosenberg, J. M. Billingsley, D. S. Colbert, N. G. Jones, A. K. Shea, A. K. Trocha, and B. D. Walker. 1999. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J. Virol. 73:6715-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalams, S. A., and B. D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188:2199.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelleher, A. D., A. Carr, J. Zaunders, and D. A. Cooper. 1996. Alterations in the immune response of human immunodeficiency virus (HIV)-infected subjects treated with an HIV-specific protease inhibitor, ritonavir. J. Infect. Dis. 173:321-329. [DOI] [PubMed] [Google Scholar]
- 28.Klein, M. R., C. A. van Baalen, A. M. Holwerda, S. R. Kerkhof Garde, R. J. Bende, I. P. Keet, J. K. Eeftinck-Schattenkerk, A. D. Osterhaus, H. Schuitemaker, and F. Miedema. 1995. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 181:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalvani, A., R. Brookes, S. Hambelton, W. J. Britton, A. V. S. Hill, and A. J. McMichael. 1998. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lane, H. C., J. M. Depper, W. C. Greene, G. Whalen, T. A. Waldmann, and A. S. Fauci. 1985. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N. Engl. J. Med. 313:79-84. [DOI] [PubMed] [Google Scholar]
- 31.Lefrere, J.-J., L. Morand-Joubert, M. Mariotti, H. Bludau, B. Burghoffer, J.-C. Petit, and F. Roudot-Throval. 1997. Even individuals considered as long-term nonprogressors show biological signs of progression after 10 years of human immunodeficiency virus infection. Blood 90:1133-1140. [PubMed] [Google Scholar]
- 32.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T-cell costimulation. Annu. Rev. Immunol. 14:233-258. [DOI] [PubMed] [Google Scholar]
- 33.Levings, M. K., and M. G. Roncarolo. 2000. T-regulatory 1 cells: a novel subset of CD4 T cells with immunoregulatory properties. J. Allergy Clin. Immunol. 106:S109-112. [DOI] [PubMed] [Google Scholar]
- 34.Li, T. S., R. Tubiana, C. Katlama, V. Calvez, H. Ait Mohand, and B. Autran. 1998. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet 351:1682-1686. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd, T. E., L. Yang, D. N. Tang, T. Bennett, W. Schober, and D. E. Lewis. 1997. Regulation of CD28 costimulation in human CD8+ T cells. J. Immunol. 158:1551-1558. [PubMed] [Google Scholar]
- 36.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moss, R. B., M. R. Wallace, W. K. Giermakowska, E. Webb, J. Savaray, C. Chamberlin-Brandt, G. Theofan, R. Musil, S. P. Richieri, F. C. Jensen, and D. J. Carlo. 1999. Phenotypic analysis of human immunodeficiency virus (HIV) type 1 cell-mediated immune responses after treatment with an HIV-1 immunogen. J. Infect. Dis. 180:641-648. [DOI] [PubMed] [Google Scholar]
- 38.Pantaleo, G., and A. S. Fauci. 1996. Immunopathogenesis of HIV infection. Annu. Rev. Microbiol. 50:825-854. [DOI] [PubMed] [Google Scholar]
- 39.Ramshaw, I. A., and A. J. Ramsay. 2000. The prime-boost strategy: exciting prospects for improved vaccination. Immunol. Today 21:163-165. [DOI] [PubMed] [Google Scholar]
- 40.Rinaldo, C., X.-L. Huang, Z. Fan, M. Ding, L. Beltz, A. Logar, D. Panicali, G. Mazzara, J. Liebmann, M. Cottrill, and P. Gupta. 1995. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J. Virol. 69:5838-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T-cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 42.Sallusto, F., C. R. Mackay, and A. Lanzavecchia. 2000. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18:593-620. [DOI] [PubMed] [Google Scholar]
- 43.Schlaak, J. F., E. Schmitt, C. Huls, K. H. Meyer zum Buschenfelde, and B. Fleischer. 1994. A sensitive and specific bioassay for the detection of human interleukin-10. J. Immunol. Methods 168:49-54. [DOI] [PubMed] [Google Scholar]
- 44.Seder, R. A., and W. E. Paul. 1994. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 12:635-673. [DOI] [PubMed] [Google Scholar]
- 45.Sloan-Lancaster, J., and P. M. Allen. 1996. Altered peptide ligand-induced partial T-cell activation: molecular mechanisms and roles in T-cell biology. Annu. Rev. Immunol. 14:1-27. [DOI] [PubMed] [Google Scholar]
- 46.Smale, S. T., and A. G. Fisher. 2002. Chromatin structure and gene regulation in the immune system. Annu. Rev. Immunol. 20:427-462. [DOI] [PubMed] [Google Scholar]
- 47.Soudeyns, H., and G. Pantaleo. 1999. The moving target: mechanisms of HIV persistence during primary infection. Immunol. Today 20:446-450. [DOI] [PubMed] [Google Scholar]
- 48.Wahren, B., L. Morfeldt-Mansson, G. Biberfeld, L. Moberg, A. Sonnerborg, P. Ljungman, A. Werner, R. Kurth, R. Gallo, and D. Bolognesi. 1987. Characteristics of the specific cell-mediated immune response in human immunodeficiency virus infection. J. Virol. 61:2017-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakkach, A., F. Cottrez, and H. Groux. 2000. Can interleukin-10 be used as a true immunoregulatory cytokine? Eur. Cytokine Netw. 11:153-160. [PubMed] [Google Scholar]
- 50.Wilson, J. D. K., N. Imami, A. Watkins, J. Gill, P. Hay, B. Gazzard, M. Westby, and F. M. Gotch. 2000. Loss of CD4+ T-cell proliferative ability but not loss of human immunodeficiency virus type 1 specificity equates with progression to disease. J. Infect. Dis. 182:792-798. [DOI] [PubMed] [Google Scholar]
- 51.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zinkernagel, R. M. 1995. Are HIV-specific CTL responses salutary or pathogenic? Curr. Opin. Immunol. 7:462-470. [DOI] [PubMed] [Google Scholar]