Abstract
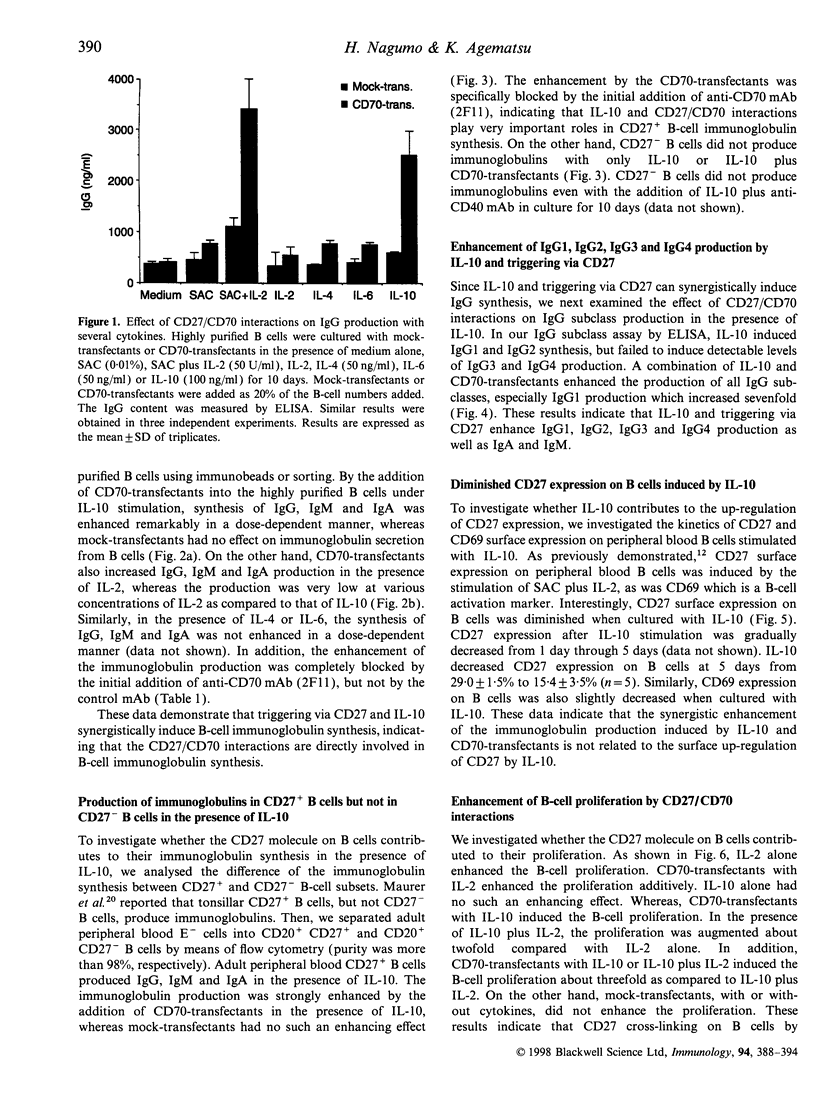
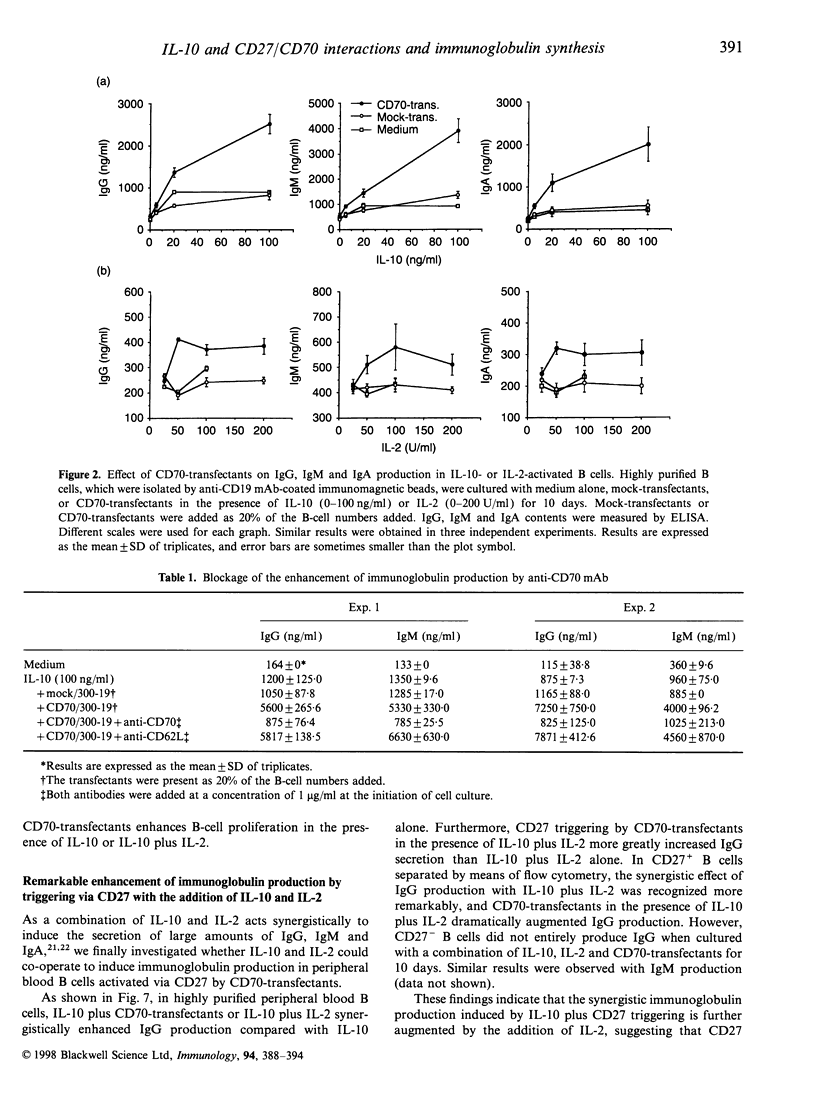
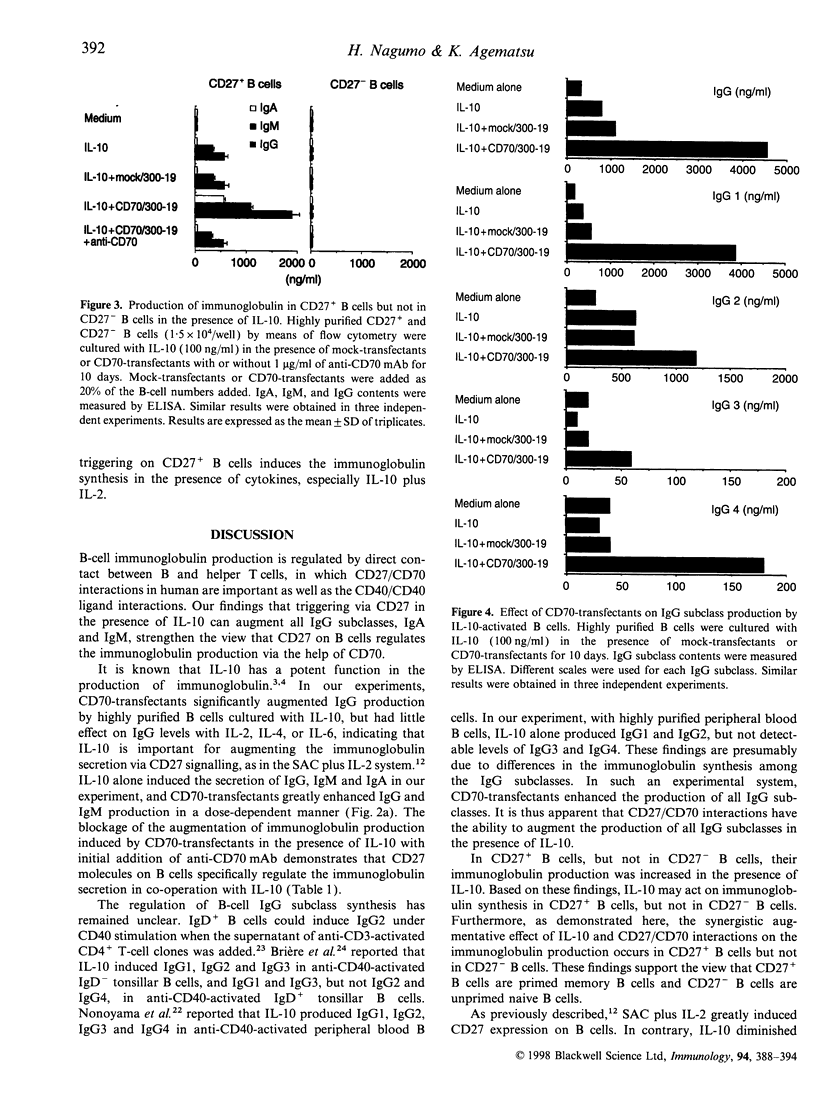
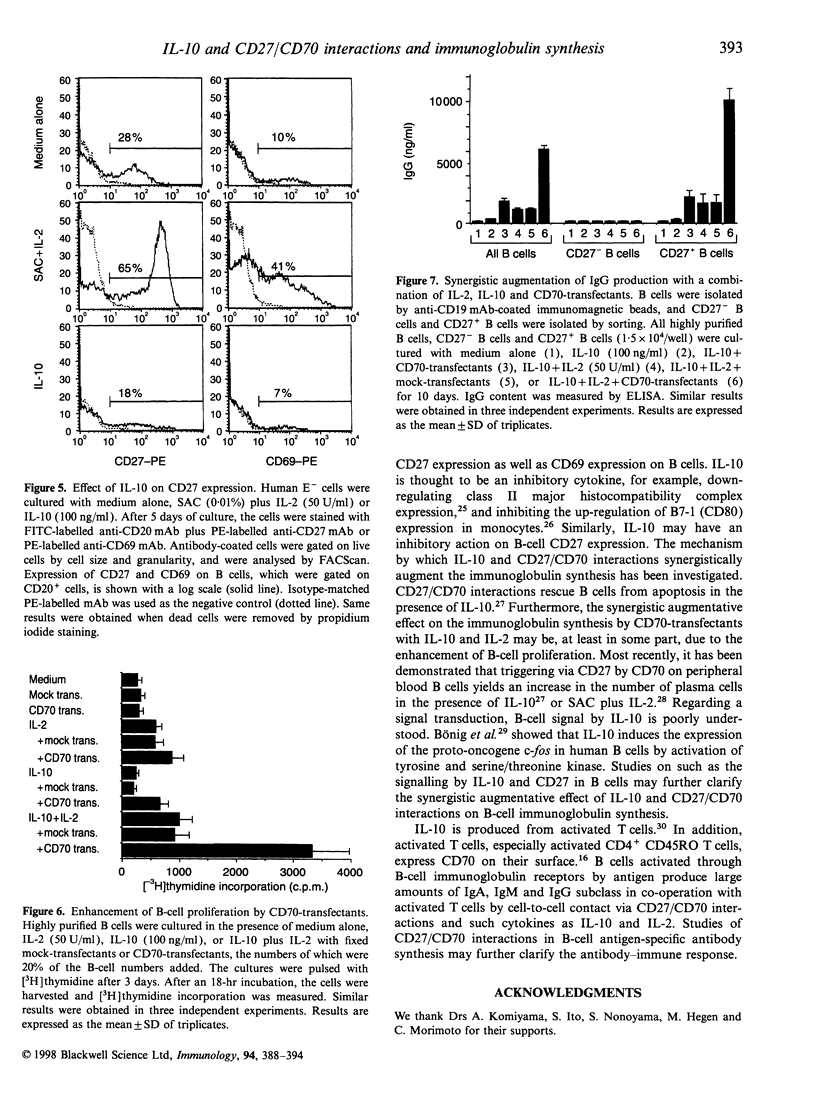
Interleukin-10 (IL-10) is a potent cytokine that regulates immunoglobulin synthesis by B cells. CD27/CD70 interactions by direct cell-to-cell contact are also needed to produce substantial amounts of immunoglobulin. We have investigated the effects of IL-10 and CD27/CD70 interactions on the immunoglobulin synthesis. In the presence of IL-10 stimulation, the production of IgG, IgM and IgA was increased synergistically by the addition of CD27 ligand (CD70)-transfectants in a dose-dependent manner, which was completely blocked by anti-CD70 monoclonal antibody. In contrast, CD70-transfectants additively enhanced the immunoglobulin production in the presence of IL-2, IL-4, or IL-6. The synergistic enhancement of the immunoglobulin production by IL-10 and CD70-transfectants was remarkable in highly purified CD27+ B cells, but there was no immunoglobulin production in CD27- B cells. Furthermore, by the addition of CD70-transfectants, the synthesis of IgG1, IgG2, IgG3 and IgG4 was also enhanced in the presence of IL-10. On the other hand, IL-10 diminished CD27 expression in B cells. B-cell proliferation was augmented by CD70-transfectants with IL-10 or IL-10 plus IL-2. The addition of IL-2 further augmented the immunoglobulin production which was synergistically enhanced by IL-10 and CD27 triggering. Taken together, the co-operative response to IL-10 and CD27/CD70 interactions regulates B-cell immunoglobulin production.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agematsu K., Kobata T., Sugita K., Hirose T., Schlossman S. F., Morimoto C. Direct cellular communications between CD45R0 and CD45RA T cell subsets via CD27/CD70. J Immunol. 1995 Apr 15;154(8):3627–3635. [PubMed] [Google Scholar]
- Agematsu K., Kobata T., Yang F. C., Nakazawa T., Fukushima K., Kitahara M., Mori T., Sugita K., Morimoto C., Komiyama A. CD27/CD70 interaction directly drives B cell IgG and IgM synthesis. Eur J Immunol. 1995 Oct;25(10):2825–2829. doi: 10.1002/eji.1830251017. [DOI] [PubMed] [Google Scholar]
- Agematsu K., Nagumo H., Oguchi Y., Nakazawa T., Fukushima K., Yasui K., Ito S., Kobata T., Morimoto C., Komiyama A. Generation of plasma cells from peripheral blood memory B cells: synergistic effect of interleukin-10 and CD27/CD70 interaction. Blood. 1998 Jan 1;91(1):173–180. [PubMed] [Google Scholar]
- Banchereau J., de Paoli P., Vallé A., Garcia E., Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991 Jan 4;251(4989):70–72. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- Bowman M. R., Crimmins M. A., Yetz-Aldape J., Kriz R., Kelleher K., Herrmann S. The cloning of CD70 and its identification as the ligand for CD27. J Immunol. 1994 Feb 15;152(4):1756–1761. [PubMed] [Google Scholar]
- Brière F., Bridon J. M., Chevet D., Souillet G., Bienvenu F., Guret C., Martinez-Valdez H., Banchereau J. Interleukin 10 induces B lymphocytes from IgA-deficient patients to secrete IgA. J Clin Invest. 1994 Jul;94(1):97–104. doi: 10.1172/JCI117354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bönig H., Körholz D., Pafferath B., Mauz-Körholz C., Burdach S. Interleukin 10 induced c-fos expression in human B cells by activation of divergent protein kinases. Immunol Invest. 1996 Jan-Mar;25(1-2):115–128. doi: 10.3109/08820139609059296. [DOI] [PubMed] [Google Scholar]
- Camerini D., Walz G., Loenen W. A., Borst J., Seed B. The T cell activation antigen CD27 is a member of the nerve growth factor/tumor necrosis factor receptor gene family. J Immunol. 1991 Nov 1;147(9):3165–3169. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. How B and T cells talk to each other. Nature. 1994 Feb 3;367(6462):425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- Ding L., Linsley P. S., Huang L. Y., Germain R. N., Shevach E. M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993 Aug 1;151(3):1224–1234. [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluckiger A. C., Garrone P., Durand I., Galizzi J. P., Banchereau J. Interleukin 10 (IL-10) upregulates functional high affinity IL-2 receptors on normal and leukemic B lymphocytes. J Exp Med. 1993 Nov 1;178(5):1473–1481. doi: 10.1084/jem.178.5.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R. G., Alderson M. R., Smith C. A., Armitage R. J., VandenBos T., Jerzy R., Tough T. W., Schoenborn M. A., Davis-Smith T., Hennen K. Molecular and biological characterization of a ligand for CD27 defines a new family of cytokines with homology to tumor necrosis factor. Cell. 1993 May 7;73(3):447–456. doi: 10.1016/0092-8674(93)90133-b. [DOI] [PubMed] [Google Scholar]
- Hintzen R. Q., Lens S. M., Beckmann M. P., Goodwin R. G., Lynch D., van Lier R. A. Characterization of the human CD27 ligand, a novel member of the TNF gene family. J Immunol. 1994 Feb 15;152(4):1762–1773. [PubMed] [Google Scholar]
- Jacquot S., Kobata T., Iwata S., Morimoto C., Schlossman S. F. CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses: enhancement of plasma cell differentiation by CD27 signaling. J Immunol. 1997 Sep 15;159(6):2652–2657. [PubMed] [Google Scholar]
- Maurer D., Fischer G. F., Fae I., Majdic O., Stuhlmeier K., Von Jeney N., Holter W., Knapp W. IgM and IgG but not cytokine secretion is restricted to the CD27+ B lymphocyte subset. J Immunol. 1992 Jun 15;148(12):3700–3705. [PubMed] [Google Scholar]
- Maurer D., Holter W., Majdic O., Fischer G. F., Knapp W. CD27 expression by a distinct subpopulation of human B lymphocytes. Eur J Immunol. 1990 Dec;20(12):2679–2684. doi: 10.1002/eji.1830201223. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Vieira P., Fiorentino D. F., Trounstine M. L., Khan T. A., Mosmann T. R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990 Jun 8;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Nonoyama S., Farrington M. L., Ochs H. D. Effect of IL-2 on immunoglobulin production by anti-CD40-activated human B cells: synergistic effect with IL-10 and antagonistic effect with IL-4. Clin Immunol Immunopathol. 1994 Sep;72(3):373–379. doi: 10.1006/clin.1994.1155. [DOI] [PubMed] [Google Scholar]
- Rousset F., Garcia E., Defrance T., Péronne C., Vezzio N., Hsu D. H., Kastelein R., Moore K. W., Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servet-Delprat C., Bridon J. M., Blanchard D., Banchereau J., Brière F. CD40-activated human naive surface IgD+ B cells produce IgG2 in response to activated T-cell supernatant. Immunology. 1995 Jul;85(3):435–441. [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Morimoto C., Schrieber M., Schlossman S. F., Saito H. Characterization of CD45 and CD45R monoclonal antibodies using transfected mouse cell lines that express individual human leukocyte common antigens. J Immunol. 1988 Dec 1;141(11):3910–3914. [PubMed] [Google Scholar]
- Sugita K., Hirose T., Rothstein D. M., Donahue C., Schlossman S. F., Morimoto C. CD27, a member of the nerve growth factor receptor family, is preferentially expressed on CD45RA+ CD4 T cell clones and involved in distinct immunoregulatory functions. J Immunol. 1992 Nov 15;149(10):3208–3216. [PubMed] [Google Scholar]
- Sugita K., Torimoto Y., Nojima Y., Daley J. F., Schlossman S. F., Morimoto C. The 1A4 molecule (CD27) is involved in T cell activation. J Immunol. 1991 Sep 1;147(5):1477–1483. [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lier R. A., Borst J., Vroom T. M., Klein H., Van Mourik P., Zeijlemaker W. P., Melief C. J. Tissue distribution and biochemical and functional properties of Tp55 (CD27), a novel T cell differentiation antigen. J Immunol. 1987 Sep 1;139(5):1589–1596. [PubMed] [Google Scholar]