Abstract
We report the complete sequence of a large rod-shaped DNA virus, called the Hz-1 virus. This virus persistently infects the Heliothis zea cell lines. The Hz-1 virus has a double-stranded circular DNA genome of 228,089 bp encoding 154 open reading frames (ORFs) and also expresses a persistence-associated transcript 1, PAT1. The G+C content of the Hz-1 virus genome is 41.8%, with a gene density of one gene per 1.47 kb. Sequence analysis revealed that a 9.6-kb region at 43.6 to 47.8 map units harbors five cellular genes encoding proteins with homology to dUTP pyrophosphatase, matrix metalloproteinase, deoxynucleoside kinase, glycine hydroxymethyltransferase, and ribonucleotide reductase large subunit. Other cellular homologs were also detected dispersed in the viral genome. Several baculovirus homologs were detected in the Hz-1 virus genome. These include PxOrf-70, PxOrf-29, AcOrf-81, AcOrf-96, AcOrf-22, VLF-1, RNA polymerase LEF-8 (orf50), and two structural proteins, p74 and p91. The Hz-1 virus p74 homolog shows high structural conservation with a double transmembrane domain at its C terminus. Phylogenetic analysis of the p74 revealed that the Hz-1 virus is evolutionarily distant from the baculoviruses. Another distinctive feature of the Hz-1 virus genome is a gene that is involved in insect development. However, the remainder of the ORFs (81%) encoded proteins that bear no homology to any known proteins. In conclusion, the sequence differences between the Hz-1 virus and the baculoviruses outnumber the similarities and suggest that the Hz-1 virus may form a new family of viruses distantly related to the Baculoviridae.
The Hz-1 virus is a nonoccluded, rod-shaped, enveloped virus with a particle size of 414 ± 30 nm (11). It contains a circular double-stranded DNA genome of 228 kb with a molecular mass of 131 × 106 Da to 140 × 106 Da (13, 34). Hz-1 virus was classified as a member of subgroup C of the Baculoviridae and is a Nudibaculoviridae species. However, low sequence homology between Hz-1 virus and other members of the Baculoviridae was observed. It has been shown that the DNA homology of the Hz-1 virus shares 3% homology with Heliothis armigera granulovirus (HearGV) and 0.1 to 1% homology with several nuclear polyhedrosis viruses (NPVs) and Plodia interpunctella granulovirus (PiGV) by Southern blot hybridization (57). Originally classified as a member of the Baculoviridae, the Hz-1 virus is currently unclassified due to its lack of an occlusion body, as well as its low DNA homology with other baculoviruses (59).
The Hz-1 virus was originally identified as a persistent viral infection in the IMC-Hz-1 cell line, which was isolated from the adult ovarian tissues of Heliothis zea (26, 44, 50). Induction of persistent Hz-1 virus was observed when the IMC-Hz-1 cells were transfected with H. zea NPV (HzNPV) DNA. This resulted in the cytopathic effect typical of a NPV but without occlusion body formation (35). Activation of the persistent Hz-1 virus in IMC-Hz-1 cells was further confirmed by infection with the homologous virus (Hz-1 virus) or the heterologous viruses HzNPV, Spodoptera frugiperda MNPV (SfMNPV), Spodoptera litura NPV (SpliNPV), and HearGV, as well as heat- and UV-inactivated Trichoplusia ni SNPV (TnSNPV) (39). The Hz-1 virus can establish persistent infections in other insect cell lines, including IPLB-Ld652 (Lymantria dispar), IPLB-Hz-1075 (Helicoverpa zea), IPLB-SF-21 (Spodoptera frugiperda), and TN368 (Tricoplusia ni) cells (32). It is the first insect virus to be reported that establishes both productive and persistent infections in insect cells (26, 39).
Temporal viral gene expression during persistent infection by Hz-1 virus has been reported (11). There are >100 genes expressed during a productive infection, with transcript sizes ranging from 0.8 to 9.5 kb (14). So far, two late genes of Hz-1 virus, p34 and p51, have been identified as expressed at 8 h postinfection during a productive Hz-1 virus infection (28, 29). In contrast, transcription of the Hz-1 viral genome is mostly silent during persistent infection, with only persistence-associated transcript 1 (PAT1) being expressed (14). The results of in situ hybridization suggest that PAT1 is localized in the nucleus. In addition, it is not associated with polysomes, and in vitro-translated products are not detected, suggesting that PAT1 is a noncoding nuclear RNA (15).
Because of the distinct temporal viral gene expression pattern in a persistent Hz-1 virus infection, the study of Hz-1 virus gene expression regulation may facilitate understanding of the switch between both productive and persistent infections, which is common in a number of viruses pathogenic for humans and animals.
We describe here the complete genome sequence of the Hz-1 virus. This analysis revealed that it has a genome with a 41.8% G+C content and contains 228,089 bp encoding 154 open reading frames (ORFs), in addition to the PAT1, with a gene density of one gene per 1.47 kb. Twenty-nine predicted Hz-1 ORFs show significant relatedness to the baculovirus and cellular genes. However, the remaining ORFs (81%) bear no detectable homology to any known proteins, suggesting that the Hz-1 virus is evolutionarily distant from the Baculoviridae.
MATERIALS AND METHODS
Virus and viral DNA.
The Hz-1 virus was serially diluted and then used to infect TN368 insect culture cells derived from the ovarian tissues of Tricoplusia ni larvae (32). The infected cells were incubated at 26°C with TNM-FH medium supplemented with 8% heat-inactivated fetal bovine serum (Gibco-BRL, Gaithersburg, Md.) for 48 to 72 h for plaque production. Hz-1 virus from single plaques was selected and scaled up in TN368 cells with a multiplicity of infection (MOI) of 0.01 PFU/cell at 26°C for 24 to 48 h. The viral titer was determined by determining the 50% tissue culture infective dose(s) (57). To extract the viral DNA, the viral suspensions were centrifuged at 800 × g (RT600D; Sorvall, Newton, Conn.) for 10 min. The supernatant was added on top of a 30% sucrose and centrifuged at 52,714 × g (SW28 rotor; Optima LE-80K Ultracentrifuge; Beckman, Fullerton, Calif.) for 30 min. The pellet was resuspended with 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) buffer and then added on top of a 40 to 65% sucrose gradient and centrifuged at 52,714 × g as described above for 1 h. The Hz-1 virus particles were banded at a 62 to 63% position. After collection into a new tube, the viral particles were suspended in 1× SSC, centrifuged twice at 52,714 × g for 30 min, and then resuspended in 1× extraction buffer (0.1 M Tris-Cl [pH 7.6], 2.5% sodium dodecyl sulfate, 0.1 M EDTA). The viral particles were digested with proteinase K (40 μg/ml; Sigma, St. Louis, Mo.) for 1 h at 50°C, and this step was followed by digestion with 50 μg of proteinase K for another 12 h. Viral genomic DNA was extracted with phenol-chloroform and precipitated with alcohol. After centrifugation, viral DNA pellets were dissolved in TE buffer (10 mM Tris [pH 8.0], 0.5 mM EDTA).
Hz-1 virus DNA cloning and sequence determination.
The Hz-1 virus was sequenced to sevenfold genomic coverage by a shotgun approach. The viral DNA was sheared by nebulization into fragments with an average size of 2,000 bp (HydroShear; GeneMachines, San Carlos, Calif.). DNA fragments were size fractionated by gel electrophoresis and cloned into the SmaI site of pBluescript SK(+/−) (Promega, Madison, Wis.). After transformation into Escherichia coli DH10B competent cells (Gibco-BRL), 3,000 recombinant colonies were picked randomly. DNA templates for sequencing were isolated by using Multiscreen kits (Millipore, Bedford, Mass.). Sequencing was performed by using the ABI Prism Big Dye Terminator Cycle Sequencing Ready reaction kit with FS AmpliTaq DNA polymerase (Perkin-Elmer, Palo Alto, Calif.) and analyzed on an ABI 377 DNA Analyzer. Shotgun sequences were base called by using the PHRED basecaller and assembled with the PHRAP assembler (22, 23). PHRAP-assembled data were stored in the Sun workstation assembly database by using the Sun workstation interface (7). The Sun workstation interface and its features were then used for editing and completing the sequence. Consensus calculations with a quality cutoff value of 40 were performed by using the Sun workstation with a probabilistic consensus algorithm based on expected error rates for output by PHRED. The PCR products bridging the ends of existing contiguous fragments were sequenced, and primers were designed to walk with genomic DNA to fill the remaining gaps in the sequence.
DNA sequence analysis.
Genomic DNA composition, structure, repeats, and restriction enzyme patterns were analyzed by using the University of Wisconsin Genetics Computer Group programs (20) and Sequencher v.4.1.2 (GeneCodes, Ann Arbor, Mich.). ORFs encoding more than 50 amino acids (150 bp) were considered protein encoding and hence were designated putative genes. The maximal 154 ORFs were predicted and analyzed from the Artemis program (http://www.sanger.ac.uk/Software/Artemis/) (54) and ORF finder and the BLAST programs of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The overlap between any two ORFs was set to a maximum of 25 amino acids. Otherwise, the largest ORF similar to the previously described ORF was selected. DNA and protein comparisons with entries in the sequence databases were performed by using the FASTA and BLAST programs (3, 50). Multiple sequence alignments were performed with the PileUp and Gap computer programs (version 10.0; Genetics Computer Group, Madison, Wis.) with the gap creation and extension penalties set to 9 and 2, respectively (20). The percent identity indicated the percentage of identical residues between two complete sequences. DNA repeats were identified by using the Miropeats computer program of EBI (European Bioinformatics Institute) (www.ebi.ac.uk/∼jparsons/packages/miropets). The presence of transmembrane (TM) domain and signal peptide (SP) in the putative ORFs were predicted by using the transmembrane hidden Markov model (TMHMM) (http://www.cbs.dtu.dk/services/TMHMM) and SignalP (http://www.cbs.dtu.dk/services/SignalP) programs of the Center for Biological Sequence Analysis, Biocentrum-DTU, Technical University of Denmark, Lyngby, Denmark. Comparison between Hz-1 virus and NPV-granulovirus genomes were performed by using the pairwise program of prFLAG (http://flag.itri.org.tw/∼cflag) from the Biomedical Engineering Center, Industrial Technology Research Center, Hsinchu, Taiwan. Phylogenetic analysis was performed by using the clustering method, UPGMA, where pairwise distance estimations were based on the proportional distance.
Nucleotide sequence accession number.
The complete Hz-1 virus sequence can be obtained from GenBank (accession no. AF451898).
RESULTS AND DISCUSSION
General features of the Hz-1 virus genome.
The Hz-1 virus genome was assembled into a contiguous sequence of 228,689 bp in good agreement with previous predictions of 228-kb based on the restriction enzyme fragment analysis and physical mapping (14). The G+C content of Hz-1 virus was 41.8%. A total of 154 ORFs, defined as methionine-initiated ORFs encoding more than 50 amino acids and with a minimal overlap with other ORFs, were present in the Hz-1 virus genome (Table 1 and Fig. 1). Together with the noncoding PAT1 (indicated by a bold arrow in Fig. 1), 155 genes were detected in the Hz-1 virus genome. The gene density of Hz-1 virus genome was one gene per 1.47 kb, much larger than that for seven NPVs and two GVs (0.87 to 0.99 kb and 0.84 to 0.99 kb, respectively) (Table 2). The ORFs were distributed evenly along the genome: 45% of them were clockwise, and 55% were anticlockwise (Fig. 2). The first ORF, Hz1V001, was defined as the first ORF present in the A fragment of XhoI-digested Hz-1 virus genome (13). The locations, orientations, sizes, and BLAST results of the ORFs are shown in Table 1. Predicted ORFs represent 68.98% coding density, with a mean ORF length of 1,015 nucleotides. The prediction of the viral capsid/coat proteins of the putative ORFs with the characteristics of the TM domain and SP was carried out with the Hidden-Markov model and SignalP software. The predicted results of ORFs with TM domain and/or SP are presented in Table 1.
TABLE 1.
Characterization of putative genes of Hz-1 virusa
| ORF | Directionb | Position
|
Lengthc
|
Promoter | BLASTP genesd | Source | BLASTP score | Identity (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | nt | aa | |||||||
| Hz1V001 | F | 620 | 3955 | 3,336 | 1,112 | L | ||||
| Hz1V002 | F | 4333 | 4503 | 171 | 57 | TM, SP | ||||
| Hz1V003 | R | 6218 | 7921 | 1,704 | 568 | E | Histidine kinase | Bacillus halodurans | 151 | 21 |
| Hz1V004 | R | 8454 | 11378 | 2,925 | 975 | E | ||||
| Hz1V005 | F | 11704 | 11922 | 219 | 73 | L | TM, SP | |||
| Hz1V006 | R | 12779 | 12931 | 153 | 51 | E | ||||
| Hz1V007 | R | 13415 | 14209 | 795 | 265 | DHFR protein kinase cyclic AMP- dependent, catalytic chain 1 | Heliothis virescens | 443 | 49 | |
| Hz1V008 | R | 14380 | 15144 | 765 | 255 | Leishmania major | 213 | 28 | ||
| Hz1V009 | F | 15274 | 16005 | 732 | 244 | |||||
| R | 16008 | 18980 | 2,973 | E | PAT1 | |||||
| Hz1V010 | F | 16094 | 16873 | 780 | 260 | PxOrf-70 | Plutella xylostella GV | 126 | 24 | |
| Hz1V011 | F | 17883 | 19931 | 2,049 | 683 | L | p74 | Spodoptera litura NPV | 402 | 22 |
| Hz1V012 | F | 20214 | 21089 | 876 | 292 | L | ||||
| Hz1V013 | F | 21412 | 22146 | 735 | 245 | L | ||||
| Hz1V014 | F | 22321 | 22491 | 171 | 57 | L | TM | |||
| Hz1V015 | F | 23171 | 23407 | 237 | 79 | |||||
| Hz1V016 | R | 23510 | 23749 | 240 | 80 | |||||
| Hz1V017 | F | 23954 | 24190 | 237 | 79 | |||||
| Hz1V018 | F | 25151 | 25309 | 159 | 53 | E | ||||
| Hz1V019 | R | 25312 | 25479 | 168 | 56 | |||||
| Hz1V020 | F | 25691 | 25846 | 156 | 52 | L | ||||
| Hz1V021 | F | 26150 | 26383 | 234 | 78 | L | TM, SP | |||
| Hz1V022 | R | 26865 | 27041 | 177 | 59 | L | ||||
| Hz1V023 | R | 27208 | 27342 | 135 | 45 | |||||
| Hz1V024 | R | 27641 | 27877 | 237 | 79 | L | ||||
| Hz1V025 | R | 28180 | 28419 | 240 | 80 | L | TM | |||
| Hz1V026 | F | 30177 | 30386 | 210 | 70 | E | ||||
| Hz1V027 | F | 30486 | 30686 | 201 | 67 | L | ||||
| Hz1V028 | F | 34670 | 35740 | 1,071 | 357 | |||||
| Hz1V029 | R | 35912 | 36850 | 939 | 313 | |||||
| Hz1V030 | F | 36914 | 37780 | 867 | 289 | TM, SP | ||||
| Hz1V031 | R | 38553 | 41990 | 3,438 | 1,146 | E | ||||
| Hz1V032 | R | 42056 | 42457 | 402 | 134 | |||||
| Hz1V033 | R | 42556 | 43026 | 471 | 157 | AcOrf-81 | Autographa californica NPV | 130 | 25 | |
| Hz1V034 | F | 43141 | 43785 | 645 | 215 | TM, SP | ||||
| Hz1V035 | F | 43954 | 44127 | 174 | 58 | L | TM, SP | |||
| Hz1V036 | R | 44312 | 48547 | 4,236 | 1,412 | DNA ligase III | Homo sapiens | 736 | 44 | |
| Hz1V037 | R | 52106 | 54937 | 2,832 | 944 | E | Probable methyl transferase | Autographa californica NPV | 311 | 35 |
| Hz1V038 | F | 55352 | 55543 | 192 | 64 | |||||
| Hz1V039 | F | 55655 | 55813 | 159 | 53 | |||||
| Hz1V040 | R | 57238 | 57432 | 195 | 65 | |||||
| Hz1V041 | R | 57601 | 58140 | 540 | 180 | |||||
| Hz1V042 | R | 58165 | 58476 | 312 | 104 | |||||
| Hz1V043 | R | 58804 | 59868 | 1,065 | 355 | E | ||||
| Hz1V044 | R | 60007 | 60282 | 276 | 92 | TM | ||||
| Hz1V045 | R | 60346 | 60546 | 201 | 67 | |||||
| Hz1V046 | R | 60720 | 63215 | 2,496 | 832 | L | vp91, viral capsid-associated protein (AcOrf-83) | Autographa californica NPV | 131 | 25 |
| Hz1V047 | F | 63587 | 65263 | 1,677 | 559 | |||||
| Hz1V048 | R | 65317 | 65502 | 186 | 62 | L | ||||
| Hz1V049 | R | 65726 | 66694 | 969 | 323 | E2 | ||||
| Hz1V050 | R | 67339 | 67734 | 396 | 132 | L | TM, SP | |||
| Hz1V051 | R | 68053 | 69963 | 1,911 | 637 | E, L | ||||
| Hz1V052 | F | 70210 | 74184 | 3,975 | 1,325 | L | ||||
| Hz1V053 | F | 74656 | 74820 | 165 | 55 | L | ||||
| Hz1V054 | R | 75184 | 75849 | 666 | 222 | |||||
| Hz1V055 | R | 76083 | 77789 | 1,707 | 569 | L | SP | |||
| Hz1V056 | R | 78048 | 78431 | 384 | 128 | |||||
| Hz1V057 | R | 78718 | 79641 | 924 | 308 | L | ||||
| Hz1V058 | F | 80420 | 82591 | 2,172 | 724 | |||||
| Hz1V059 | R | 83062 | 84294 | 1,233 | 411 | |||||
| Hz1V060 | F | 84435 | 87065 | 2,631 | 877 | |||||
| Hz1V061 | F | 87339 | 87593 | 255 | 85 | L | ||||
| Hz1V062 | R | 88323 | 92270 | 3,948 | 1,316 | E, L | ||||
| Hz1V063 | R | 92396 | 92950 | 555 | 185 | L | TM | |||
| Hz1V064 | R | 93091 | 94242 | 1,152 | 384 | HL | p51 | Macaca mulatta rhadinovirus 17577 | 95 | 21 |
| Hz1V065 | R | 94271 | 94444 | 174 | 58 | L | TM, SP | |||
| Hz1V066 | F | 94610 | 94948 | 339 | 113 | |||||
| Hz1V067 | F | 95097 | 96485 | 1,389 | 463 | L | ||||
| Hz1V068 | F | 96560 | 98518 | 1,959 | 653 | |||||
| Hz1V069 | F | 99357 | 100409 | 1,053 | 351 | dUTP pyrophosphatase | Homo sapiens | 386 | 58/PICK> | |
| Hz1V070 | R | 101531 | 103909 | 2,379 | 793 | Matrix metalloproteinase | Mus musculus | 464 | 29 | |
| Hz1V071 | R | 104121 | 104945 | 825 | 275 | Deoxynucleoside kinase | Drosophila melanogaster | 397 | 41 | |
| Hz1V072 | F | 106014 | 107339 | 1,326 | 442 | Glycine hydroxymethyltransferase | Drosophila melanogaster | 1434 | 61 | |
| Hz1V073 | R | 107960 | 108961 | 1,002 | 334 | rr2 | Equine herpesvirus 1 | 1150 | 67 | |
| Hz1V074 | R | 109072 | 109536 | 465 | 155 | TM | ||||
| Hz1V075 | F | 109742 | 113350 | 3,609 | 1203 | E | DNA-directed RNA polymerase | Yeast (Kluyveromyces marxianus var. lactis) | 116 | 26 |
| Hz1V076 | F | 113737 | 115065 | 1,329 | 443 | TM | Plasmid pSKL | |||
| Hz1V077 | R | 115274 | 116017 | 744 | 248 | |||||
| Hz1V078 | R | 116225 | 116599 | 375 | 125 | L | ||||
| Hz1V079 | R | 116876 | 117640 | 765 | 255 | E, L, HL | p34 | Hz-1 insect virus | 1308 | 100 |
| Hz1V080 | R | 117679 | 117840 | 162 | 54 | L | ||||
| Hz1V081 | F | 117933 | 118313 | 381 | 127 | E, HL | TM, SP | |||
| Hz1V082 | F | 118605 | 118793 | 189 | 63 | L | TM, SP | |||
| Hz1V083 | R | 122431 | 123837 | 1,407 | 469 | L | ||||
| Hz1V084 | F | 124009 | 124212 | 204 | 68 | |||||
| Hz1V085 | R | 124410 | 124694 | 285 | 95 | L | ||||
| Hz1V086 | R | 124758 | 124931 | 174 | 58 | SP | ||||
| Hz1V087 | F | 124951 | 125394 | 444 | 148 | |||||
| Hz1V088 | R | 125907 | 126548 | 642 | 214 | PxOrf-29 | Plutella xylostella GV | 96 | 29 | |
| Hz1V089 | R | 127093 | 127938 | 846 | 282 | L | ||||
| Hz1V090 | F | 128075 | 131866 | 3,792 | 1,264 | L | RNA polymerase LEF-8 (Orf-50) | Bombyx mori NPV | 101 | 24 |
| Hz1V091 | F | 132319 | 132498 | 180 | 60 | |||||
| Hz1V092 | F | 132556 | 132921 | 366 | 122 | |||||
| Hz1V093 | R | 133290 | 134036 | 747 | 249 | |||||
| Hz1V094 | F | 134193 | 134555 | 363 | 121 | |||||
| Hz1V095 | R | 134950 | 137769 | 2,820 | 940 | L | rr1 | Equine herpesvirus 1 | 2137 | 53 |
| Hz1V096 | R | 138005 | 139477 | 1,473 | 491 | E | ||||
| Hz1V097 | F | 139781 | 140824 | 1,044 | 348 | |||||
| Hz1V098 | F | 141233 | 143899 | 2,667 | 889 | |||||
| Hz1V099 | R | 145139 | 145900 | 762 | 254 | |||||
| Hz1V100 | R | 145986 | 146882 | 897 | 299 | |||||
| Hz1V101 | F | 147162 | 147887 | 726 | 242 | L | ||||
| Hz1V102 | R | 148545 | 148715 | 171 | 57 | E | ||||
| Hz1V103 | R | 148816 | 150930 | 2,115 | 705 | AcOrf-96 | Xestia c-nigrum GV | 126 | 23 | |
| Hz1V104 | F | 150932 | 155689 | 4,758 | 1,586 | |||||
| Hz1V105 | F | 156572 | 156727 | 156 | 52 | |||||
| Hz1V106 | R | 156774 | 157796 | 1,023 | 341 | L | ||||
| Hz1V107 | F | 158399 | 162130 | 3,732 | 1,244 | |||||
| Hz1V108 | R | 162963 | 163127 | 165 | 55 | |||||
| Hz1V109 | R | 163181 | 164056 | 876 | 292 | E,L | ts | Mus musculus | 1064 | 70 |
| Hz1V110 | F | 164264 | 164440 | 177 | 59 | L | TM | |||
| Hz1V111 | R | 164454 | 165461 | 1,008 | 336 | |||||
| Hz1V112 | F | 165694 | 166974 | 1,281 | 427 | E | ||||
| Hz1V113 | R | 168405 | 168560 | 156 | 52 | |||||
| Hz1V114 | F | 168579 | 168740 | 162 | 54 | TM, SP | ||||
| Hz1V115 | R | 169103 | 170377 | 1,275 | 425 | L | ||||
| Hz1V116 | R | 170418 | 170615 | 198 | 66 | |||||
| Hz1V117 | F | 171120 | 171602 | 483 | 161 | E, L | SP | |||
| Hz1V118 | R | 172592 | 173560 | 969 | 323 | |||||
| Hz1V119 | F | 173602 | 176073 | 2,472 | 824 | TM, SP | ||||
| Hz1V120 | F | 176098 | 176286 | 189 | 63 | |||||
| Hz1V121 | F | 176883 | 177884 | 1,002 | 334 | E | VLF-1 | Spodoptera exigua NPV | 84 | 56 |
| Hz1V122 | R | 178281 | 180080 | 1,800 | 600 | |||||
| Hz1V123 | R | 180164 | 181306 | 1,143 | 381 | AcOrf-22 | Bombyx mori NPV | 208 | 26 | |
| Hz1V124 | R | 181949 | 182278 | 330 | 110 | L | TM, SP | |||
| Hz1V125 | R | 182341 | 182769 | 429 | 143 | L | ||||
| Hz1V126 | R | 183078 | 184616 | 1,539 | 513 | CG4526 gene product (alt1) | Drosophila melanogaster | 1140 | 48 | |
| Hz1V127 | R | 184654 | 185178 | 525 | 175 | |||||
| Hz1V128 | F | 185280 | 187370 | 2,091 | 697 | E | ||||
| Hz1V129 | F | 188310 | 188741 | 432 | 144 | |||||
| Hz1V130 | R | 189943 | 190356 | 414 | 138 | L | ||||
| Hz1V131 | F | 190540 | 193950 | 3,411 | 1,137 | L | DNA polymerase I | Aeropyrum pernix | 124 | 23 |
| Hz1V132 | F | 194777 | 195013 | 237 | 79 | L | TM, SP | |||
| Hz1V133 | F | 195093 | 195281 | 189 | 63 | L | ||||
| Hz1V134 | R | 196267 | 197592 | 1,326 | 442 | E | ||||
| Hz1V135 | R | 197755 | 198303 | 549 | 183 | E | Inhibitor of apoptosis protein | Orgyia pseudotsugata NPV | 309 | 35 |
| Hz1V136 | R | 199229 | 200053 | 825 | 275 | |||||
| Hz1V137 | R | 202089 | 203438 | 1,350 | 450 | E | ||||
| Hz1V138 | R | 203887 | 204453 | 567 | 189 | IAP | Buzura suppressaria NPV | 290 | 37 | |
| Hz1V139 | R | 204781 | 204945 | 165 | 55 | L | ||||
| Hz1V140 | F | 205208 | 207643 | 2,436 | 812 | E, L | ||||
| Hz1V141 | F | 207769 | 210198 | 2,430 | 810 | |||||
| Hz1V142 | F | 210245 | 210493 | 249 | 83 | |||||
| Hz1V143 | F | 211082 | 211276 | 195 | 65 | E | SP | |||
| Hz1V144 | F | 211278 | 212285 | 1,008 | 336 | |||||
| Hz1V145 | F | 213258 | 215354 | 2,097 | 699 | Carboxylesterase | Aphis gossypii | 226 | 32 | |
| Hz1V146 | R | 215729 | 215887 | 159 | 53 | L | ||||
| Hz1V147 | R | 216178 | 216375 | 198 | 66 | TM, SP | ||||
| Hz1V148 | R | 216788 | 217228 | 441 | 147 | |||||
| Hz1V149 | R | 217623 | 217796 | 174 | 58 | |||||
| Hz1V150 | F | 217831 | 218781 | 951 | 317 | |||||
| Hz1V151 | R | 220142 | 220846 | 705 | 235 | L | ||||
| Hz1V152 | F | 221294 | 221458 | 165 | 55 | E | MP | |||
| Hz1V153 | R | 221867 | 222625 | 759 | 253 | |||||
| Hz1V154 | R | 222659 | 227176 | 4,518 | 1,506 | |||||
Nucleotides in the Hz-1 virus genome were numbered sequentially, beginning with the nucleotide in the XhoI-A fragment. ORFs in the Hz-1 virus genome over 150 bp in length were designated ORF1 to ORF154, beginning with the first ORF in the XhoI-A fragment. PAT1 is the noncoding RNA that is transcribed from the reverse strand between the Hz1 V010 and Hz1V011. The presence of baculovirus early (E and E2) and late (L) promoter elements, located within 300 nucleotides of the ATG, is indicated. E and E2 indicate TATAA sequence with a CA(T/G)T (E) or CGTGC (E2) start site sequence 20 to 40 nucleotides downstream. L indicates the presence of a (A/T/G)TAAG motif. In addition, the promoter scanning was also carried out for detecting the Hz-1 virus late (HL) promoter sequence TTATAGTAT. The position of each ORF defines the A the initiation codon (ATG) and the T of the termination codon (TAA/TAG/TGA) of its encoding strand.
The directions of the transcripts are indicated by F (forward) or R (reverse).
nt, nucleotides; aa, amino acids.
TM, TM domain gene; SP, SP gene.
FIG. 1.
Circular representation of the Hz-1 virus genome. The arrows indicate the positions (outer ring) of the 154 ORFs and the noncoding PAT1. X, sites of XhoI restriction enzymes (inner ring [positions are indicated in parentheses]). The repeated regions (R1, R2, and R3) are indicated in the innermost ring. The position of PAT1 is indicated by a heavy arrow.
TABLE 2.
Comparisons of the genome of Hz-1 virus and various baculoviruses
| Virus | Genome size | No. of genes | G+C content (%) | Gene density (kb) | No. of HRs | Source or reference |
|---|---|---|---|---|---|---|
| Hz-1 | 228108 | 155 | 41.8 | 1.47 | NDa | This study |
| LdMNPV | 161046 | 163 | 57.5 | 0.99 | 13 | 43 |
| SeMNPV | 135611 | 139 | 44.0 | 0.98 | 6 | 36 |
| AcMNPV | 133894 | 154 | 41.0 | 0.87 | 8 | 5 |
| OpMNPV | 131990 | 152 | 55.0 | 0.87 | 5 | 3 |
| HearNPV | 131403 | 135 | 39.1 | 0.97 | 5 | 17 |
| BmNPV | 128413 | 136 | 40.0 | 0.94 | 7 | 25 |
| CuniNPV | 108252 | 109 | 50.9 | 0.99 | 4 | 2 |
| XecnGV | 178733 | 181 | 40.7 | 0.99 | 8 | 31 |
| PxGV | 100999 | 120 | 40.7 | 0.84 | 4 | 30 |
ND, no homologous regions were detected in the Hz-1 virus genome.
FIG. 2.
Layout of the genes and elements in the Hz-1 virus genome. The genome is shown expanded from the representation in Fig. 1. The protein-coding regions and orientations for the recognized genes are as listed in Table 1. The repeated regions (R1, R2, and R3) are indicated.
A total of 24 Hz-1V ORFs possessed a consensus early promoter motif (a TATA box followed by a CAG/TT motif located 20 to 25 bp downstream) within 180 bp (41) of the initiation codon (Table 1). Of these, five ORFs also possessed a late gene promoter motif, which may allow transcription of theses genes during both early and late stages of infection. A total of 45 Hz-1V ORFs possessed a consensus late gene promoter motif within 160 bp of the initiation codon. One Hz-1V ORF was found that contained a CGTGC motif that has also been identified as an early promoter consensus sequence-transcription initiation site (41). A 9-bp sequence of TTATAGTAT was identified at the upstream regulatory regions of both p34 and p51 late genes of Hz-1 virus (28, 29). A TTATAGTAT motif was found within 200 bp of the initiation codon of three Hz-1V ORFs, including p34 and p51. Of the Hz-1V ORFs, 80 did not possess consensus late or early promoter sequences. It is possible that they may be transcribed from unique early promoters or from consensus late gene promoters other than these sequences.
Repeated regions of Hz-1 virus.
The baculovirus contains homologous regions (HRs) containing AT-rich sequences of direct repeats, as well as inverse repeats that serve as the origins for DNA replication (49) and enhancers of early transcription (27). Homologous regions have been identified in all baculovirus genomes sequenced to date, including AcMNPV (5), OpMNPV (3), BmNPV (25), LdMNPV (43a), and CuniNPV (2). It has been considered that the presence of HRs is a characteristic feature of the baculoviruses. However, no HRs were identified in the Hz-1 virus genome. Instead, abundant tandem sequence repeats of 21 to 75 bp were identified throughout the Hz-1 virus genome. There are highly repeated sequences identified at positions 57243 to 58675 (R1), 59090 to 59359 (R2), and 105345 to 105885 (R3) of the Hz-1 virus genome (Fig. 1).
Hz-1 virus ORFs encoding products homologous to known proteins.
Among the 154 ORFs identified, only 29 have significant homologies to the current database. These include nine involved in nucleic acid metabolism, two involved in DNA replication, three involved in gene regulation, one virion, one nucleocasid protein, and p34 (product of Hz1V079) and p51 (product of Hz1V064), which are the Hz-1 virus late genes as previously reported (28, 29) (Table 1). However, 125 ORFs (81%) have no similarities to any known genes, suggesting that the Hz-1 virus may form a novel class of virus that is distant from the baculoviruses.
Enzymes involved in nucleic acid metabolism.
Nine genes—Hz1V003, Hz1V007, Hz1V037, Hz1V043, Hz1V069, Hz1V071, Hz1V073, Hz1V095, and Hz1V109—show extensive homologies with previously identified proteins and therefore may encode the Hz-1 virus homologues of enzymes involved in nucleic acid metabolism. The most significant homology (70% identity over 292 amino acids) was detected between the product of Hz1V109 and the human thymidylate synthase gene (ts) (Table 1). Thymidylate synthase catalyzes the metabolism of dUTP to yield the nucleotide precursor of dTMP and is an important step in the de novo pathway of biosynthesis of pyrimidine (12). So far, no ts gene has been reported for baculoviruses. However, homologs of the ts gene have been identified in the genomes of two other insect viruses, Chilo iridescent virus (47) and Melanoplus sanguinipes entomopoxvirus (1). Our previous analysis suggested that the Hz-1 virus ts gene originated from its lepidopteran host and that the ts genes of Hz-1 virus, Chilo iridescent virus, and Melanoplus sanguinipes entomopoxvirus originated from independent recombination events (16).
The products of Hz1V095 and Hz1V073 show significant homologies to both the large (rr1) and small (rr2) subunits of ribonucleotide reductase (53% identity to equine herpesvirus rr1 over 940 amino acids, and 67% identity to equine herpesvirus rr2 over 334 amino acids, respectively). The rr1 and rr2 genes were separated by 25,989 bp. This enzyme is involved in nucleotide metabolism and reduces ribonucleotides into deoxyribonucleotides as immediate precursors of DNA (37). Hz1V069 is homologous to dUTP pyrophosphatase (dUTPase) with a 58% identity to Homo sapiens dUTPase over 351 amino acids. dUTPase cleaves the alpha-beta phosphodiester bonds of dUTP to form pyrophosphate and dUMP, preventing incorporation of uracil into DNA and providing the substrate for thymine synthesis (42). dUTPase has been shown to be essential for the replication of a number of DNA viruses (6). Hz1V071 shows a 44% identity to deoxynucleoside kinase of Drosophila melanogaster over 275 amino acids. Deoxynucleoside kinases are key enzymes in deoxyribonucleoside salvage (37). Hz1V007 shows a 49% identity to dihydrofolate reductase (DHFR) of Heliothis virescens over 265 amino acids. Hz1V037 shows a 35% identity to the rRNA methyltransferase J large subunit of AcMNPV over 944 amino acids. Hz1V003 shows a 21% identity to histidine kinase of Bacillus halodurans over 568 amino acids (Table 1).
The presence of nucleotide metabolism enzymes suggests that the Hz-1 virus may synthesize its nucleotides independently of the host cell machinery. A number of viral genes could be acquired from genome DNA or from another virus infecting a common host (53). Molecular mimicry or genetic piracy, with respect to the utilization of cellular genes captured and modified during the course of viral evolution, has been an area of increasing research with the expansion in virus genome sequencing (19). A common feature of these captured genes is that they are nonessential for virus replication in vitro and that they confer a selective advantage for virus replication, persistence, and spread or in dealing with host cell differentiation and immune defense mechanisms in vivo (9, 10, 47, 55). However, few cases have reported on a viral gene captured from its insect host, although many viruses can infect insects.
Proteins involved in DNA replication and transcription.
Two genes involved in DNA replication were identified in the Hz-1 virus genome. Hz-1 virus DNA ligase (product of Hz1V036) shows a 44% identity to that of Homo sapiens over 1,412 amino acids. The Hz-1 virus DNA polymerase (a product of Hz1V131) was putatively identified by the presence of three highly conserved motifs that are found in most eukaryotic DNA polymerases gene, as well as in some viral polymerases. Among the three homologues to DNA transcription genes, Hz1V075 shows a 26% identity to yeast (Saccharomyces kluyveri) plasmid pSKL encoding DNA-directed RNA polymerase (33) over 1,203 amino acids. Hz1V090 shows a 24% identity to DNA-dependent RNA polymerase LEF-8 (orf50) of BmNPV over 1,264 amino acids (Table 1). Hz1V121 shows a high homology to VLF-1 (56% identity to that of SeMNPV over 334 amino acids). VLF-1 has been shown to regulate very late gene expression (45) and also plays a crucial role in the replication of the budded virus form of AcMNPV (60).
Structural proteins.
Among the 29 ORFs of Hz-1 virus that show significant homologies to known proteins, two ORFs show significant homologies to two of baculovirus structural proteins: p74 and vp91-capsid associated protein (products of Hz1V011 and Hz1V046, respectively). No homologs of gp64/ld130 groups were identified in the predicted Hz-1V ORFs. Hz1V012 shows a 21 to 23% amino acid identity to p74 of 9 NPVs, 2 GVs, and Culex nigipalpus NPV. The amino acid sequences of the p74 gene homologs among the 12 baculoviruses ranged from 31 to 91% identity (56). It has been shown that p74 is localized on the outside of the virion envelope and is important for virus entry into insect midgut cells (24). The N terminus of p74 is exposed on the ODV surface and the C terminus of p74 acts as a TM anchor (24). A remarkable double TM domain at the C terminus of p74 was detected among the 12 baculoviruses and the Hz-1 virus by using the Hidden-Markov model. However, a region with a high amino acid sequence similarity between the Hz-1 virus p74 and that in the baculoviruses was located between amino acids 7 and 579, upstream of the double TM domain (amino acids 624 to 646 and amino acids 673 to 682) (Fig. 3). It has been suggested that the C terminus of p74 directs the protein into the envelope that surrounds occluded virions (56). Consistent with this hypothesis, the deletion of the C terminus of AcMNPV p74 abolishes insect oral infectivity but does not interfere with virus replication in cell cultures (43). No occlusion bodies (OBs) were detected for the Hz-1 virus in cell cultures.
FIG. 3.
Multiple amino acid sequence alignment of Hz-1 virus p74 homolog (product of Hz1V011) with the p74 of BmNPV (NP_047536.1), AcMNPV (NP_054168.1), OpMNPV (U75930), CfNPV (M97904), LdMNPV (NP_047663.1), HearNPV (NP_075089.1), Spodoptera litura NPV (SpltNPV) (AJ01155858), XecnGV (NP_059225.1), Leucania separata NPV (LsNPV) (AB009455), and CuniNPV (AF274288). Identity with consensus is denoted by black box. Similarity with the consensus is denoted by gray shading, differences are indicated by white, and gaps in the alignment are indicated by dots. The positions of the amino acid sequences are indicated on the right.
The Hz1V046 product shows a 35% amino acid identity to vp91 capsid-associated protein, a baculovirus capsid-associated protein (5). In both Hz1V046 and the vp91 capsid-associated protein, a region containing chitin-binding peritrophin A domain (amino acids 250 to 305) was revealed by PFAM searches (Fig. 3).
A continuous 9.6-kb locus contains five homologues of cellular genes.
A continuous 9.6-kb sequence located at 43.6 to 47.8 map units contained five genes homologous to cellular genes (Hz1V069 to Hz1V073). The Hz1V070 product shows 29% amino acid sequence identity to Mus musculus matrix metalloproteinase (MMP) over 185 amino acids. An MMP gene was also detected in the Xestia c-nigrum GV (XecnGV) genome and encodes an ORF of 469 amino acids that has the conserved catalytic domains of human MMP3 (40). MMP homologs have not been reported for other insect viruses. The Hz1V072 product shows a 61% amino acid identity to Drosophila melanogaster serine hydroxymethyltransferase (SHMT). It has been suggested that thymidylate synthase and DHFR, along with SHMT, form a metabolic cycle that methylates dUMP to dTMP. The SHMT enzyme catalyzes the THF-dependent reversible conversion of serine to glycine (18).
The acquisition of several continuous cellular proteins has been observed in Kaposi sarcoma-associated herpesvirus (KSHV, also named human herpesvirus 8) (48). A single 13-kb locus in the KSHV genome contains nine ORFs that are homologous to or related to cellular proteins (48). These include a complete ts gene, a DHFR gene, four novel cytokine genes, and a bcl-2 homologue (48).
Other cellular homologs.
The Hz1V126 product encodes an ORF of 513 amino acids that shows a 48% amino acid identity to CG4526 of Drosophila melanogaster which harbors a sugar transporter domain from amino acids 53 to 508. Interestingly, the Hz1V145 product encodes an ORF of 699 amino acids that shows a 32% amino acid identity to Drosophila melanogaster juvenile hormone esterase (JHE) that is involved in insect morphogenesis (52). The Hz1V-JHE contains the conserved carboxylesterase domain from amino acids 3 to 453. No JHE homologues have been detected in other insect viruses. JHE inactivates juvenile hormone, which regulates the outcome of an insect molt, and is an essential enzyme for normal insect development. It has been an attractive targets for biorationally designed, environmentally safe pesticides (8, 21). The expression of JHE by the Hz-1 virus may influence the developmental characteristics, weight gain, and time of mortality of the insects.
Comparison of the Hz-1 virus ORFs with baculovirus ORFs.
The complete sequences of five NPVs and two GVs pathogenic for Lepidoptera, including AcMNPV, BmNPV, OpMNPV, SeMNPV, LdMNPV, PlxyGV, and XecnGV, yielded a wealth of information relating to the gene structure and organization of baculoviruses. A comparison of the Hz-1 virus gene content to that of the baculoviruses (31) shows that some Hz-1 virus genes are conserved in baculoviruses, including 11 genes involved in DNA replication, transcription, structural proteins, and the regulation of host metabolism, and five unknown baculovirus homologues (Table 3). However, the most conserved baculovirus gene, polyhedrin or granulin, was not identified in the Hz-1 virus genome.
TABLE 3.
Baculovirus gene homologs present in the Hz-1 virus genome
| Gene type | Conserved genesa | Variable genesb |
|---|---|---|
| Replication genes | dna-pol | rr1, rr2, dna-lig, dutpase |
| Transcription-specific genes | vlf-1 | |
| Structural protein genes | p74, vp91-capsid, pk-1 | |
| Genes homologs that may alter host metabolism | iap1, iap2 | |
| Unknown | AcOrf-22, AcOrf-81, AcOrf-96, PxOrf-29, PxOrf-70 |
Conserved genes are identified in all the eight baculoviruses.
Variable genes are identified in some of the eight baculoviruses.
Further comparison of the Hz-1 virus genome with the NPVs and GVs genomes was carried out by using the prFLAG pairwise program. The results showed that no significant similarity was found either between the Hz-1 virus and AcMNPV, HearNPV, PlxyGV, and XecnGV genomes (data not shown). Interestingly, the low similarity between the NPVs and GVs suggests that the Hz-1 virus is at least a different genus from these groups.
Phylogeny of Hz-1 virus.
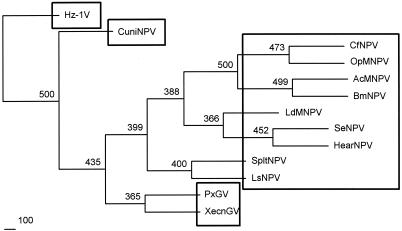
Although the Hz-1 virus has been excluded from the Baculoviridae, the data from our sequence analysis indicate a striking similarity, including 16 genes that appear to be shared between the two groups. These include baculovirus homologs that are conserved for DNA replication, gene expression regulation, structural genes, and genes that affect the host metabolism of baculoviruses. However, the lack of homologous regions within the entire Hz-1 virus genome, an overall low level of identity between ORFs, the lack of conserved gene order, the absence of many genes present in all lepidopteran baculovirus genomes, and the lack of polyhedrin/granulin and p10 homologs all suggest that the Hz-1 virus is distantly related to the baculoviruses. Recently, a similar situation has been reported for the Culex nigripalpus NPV (CuniNPV), which is a pathogenic baculovirus for dipteran, except that it has four putative hrs (2, 46). CuniNPV has globular OBs and is not enveloped (46). The phylogenetic analysis of CuniNPV p74 showed that it is a member of the baculovirus lineage distinct from lepidopteran NPVs and GVs (46). The phylogenetic analysis of Hz-1 virus p74 suggests that it is even further away from the NPVs and GVs than CuniNPV is (Fig. 4). The Hz-1 virus and CuniNPV genomic sequence analyses results suggest that these viruses may have gone through different evolution pathways from those of the NPVs and GVs. However, unlike CuniNPV, neither hrs nor OBs were identified in the Hz-1 virus genome. In conclusion, our analysis of the Hz-1 virus genome sequence revealed that the differences outnumber the similarities between the Hz-1 virus and baculoviruses and suggest that the Hz-1 virus may form a different class of virus that is distinct from Baculoviridae. Further comparison of the Hz-1 virus with other nudiviruses and baculoviruses should provide important insights into the evolution of both invertebrate and vertebrate viruses.
FIG. 4.
Phylogenetic analysis of the p74 gene based on the amino acid distances from a variety of organisms. The trees were constructed by using a UPGMA clustering method. Bootstrap values of >50 are shown above the node in 500 replications.
Acknowledgments
We thank A. Yaw, P. I. Huang, and K. H. Liang of the Biomedical Engineering Center, Industrial Technology Research Institute, Hsinchu, Taiwan, for assistance with prFLAG. We thank H. R. Cheng and T. W. Hsu for excellent technical assistance.
This work was supported by grant NSC89-2311-B-006-003 from the National Science Council of Taiwan.
REFERENCES
- 1.Afonso, C. L., E. R. Tulman, Z. Lu, E. Oma, G. F. Kutish, and D. L. Rock. 1999. The genome of Melanoplus sanguinipes entomopoxvirus. J. Virol. 73:533-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afonso, C. L., E. R. Tulman, A. Lu, C. A. Balinsky, B. A. Moser, J. J. Becnel, D. L. Rock, and G. F. Kutish. 2001. Genome sequence of a baculovirus pathogenic for Culex nigripalpus. J. Virol. 75:11157-11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahrens, C. H., R., Russell, C. J. Funk, J. T. Evans, S. H. Harwood, and G. F. Rohrmann. 1997. The sequence of the Orygia pseudotsugata multinucleocapsid nuclear polyhedrosis virus genome. Virology 229:381-399. [DOI] [PubMed] [Google Scholar]
- 4.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 5.Ayres, M. D., S. C. Howard, J. Kuzio, M. Lopez-Ferber, and R. D. Possee. 1994. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology 202:586-605. [DOI] [PubMed] [Google Scholar]
- 6.Baldo, A. M., and M. A. McClure. 1999. Evolution and horizontal transfer of dUTPase-encoding genes in viruses and their hosts. J. Virol. 73:7710-7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonfield, J. K., K. F. Smith, and R. Staden. 1995. A new DNA sequence assembly program. Nucleic Acids Res. 23:4992-4999. [DOI] [PMC free article] [PubMed]
- 8.Bonning, B. C., P. W. Roelvink, J. M. Vlak, R. D. Possee, and B. D. Hammock. 1994. Superior expression of juvenile hormone esterase and beta-galactosidase from the basic promoter of Autographa california nuclear polyhedrosis virus compared to the p10 protein and polyhedrin promoters. J. Gen. Virol. 75:1551-1556. [DOI] [PubMed] [Google Scholar]
- 9.Brazas, R., and D. Ganem. 1996. A cellular homolog of hepatitis delta antigen: implications for viral replication and evolution. Science 274:90-94. [DOI] [PubMed] [Google Scholar]
- 10.Bugert, J. J., and G. Darai. 2000. Poxvirus homologues of cellular genes. Virus Genes 21:111-133. [PubMed] [Google Scholar]
- 11.Burand, J. P., C. Y. Kawanishi, and Y. S. Huang. 1986. Persistent baculovirus infection, p. 159-175. In R. R. Granados and B. A. Fedderici (ed.), The biology of baculovirus. CRC Press, Inc., Boca Raton, Fla.
- 12.Carreras, C. W., and D. V. Santi. 1995. The catalytic domain and structure of thymidylate synthase. Annu. Rev. Biochem. 64:721-762. [DOI] [PubMed] [Google Scholar]
- 13.Chao, Y. C., M. Hamblin, and H. A. Wood. 1990. Physical map of Hz-1 baculovirus genome from standard and defective interfering particles. J. Gen. Virol. 71:1265-1270. [Google Scholar]
- 14.Chao, Y. C., H. A. Wood, C. Y. Chang, H. J. Lee, W. C. Shen, and H. T. Lee. 1992. Different expression of Hz-1 baculovirus genes during productive and persistent infections. J. Virol. 63:1442-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao, Y. C., S. T. Lee, M. C. Chang, H. H. Chen, S. S. Chen, T. Y. Wu, F. H. Liu, E. L. Hsu, and R. F. Hou. 1998. A 2.9-kilobase noncoding nuclear RNA functions in the establishment of persistent Hz-1 viral infection. J. Virol. 72:2233-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, H. H., D. J. Tso, W. B. Yeh, H. J. Cheng, and T. F. Wu. 2001. The thymidylate synthase gene of Hz-1 virus: a gene captured from its lepidopteran host. Insect Mol. Biol. 10:495-503. [DOI] [PubMed] [Google Scholar]
- 17.Chen, X., M. Li, X. Sun, B. M. Arif, Z. Hu, and J. M. Vlak. 2000. Genomic organization of Helicoverpa armigera single-nucleocapsid nucleopolyhedrovirus. Arch. Virol. 45:2539-2555. [DOI] [PubMed] [Google Scholar]
- 18.Chu, E., and C. J. Allegra. 1996. The role of thymidylateee synthase in cellular regulation. Bioessays 18:191-198.8867733 [Google Scholar]
- 19.Davis-Poynter, N. J., M. Degli-Espsti, and H. E. Farrell. 1999. Murine cytomegalovirus homologues of cellular immunomodulatory genes. Intervirology 42:331-341. [DOI] [PubMed] [Google Scholar]
- 20.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed]
- 21.Eldridge, R., D. R. O'Relly, B. D. Hammock, and L. K. Miller. 1992. Insecticidal properties of genetically engineered baculoviruses expressing an insect juvenile hormone esterase gene. Appl. Environ. Microbiol. 58:1583-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ewing, B., and P. Green. 1998. Base-calling of automated sequencer traces using PHRED. II. Error probabilities. Genome Res. 8:186-194. [PubMed] [Google Scholar]
- 23.Ewing, B., L. Hillier, and M. C. Wendl, and P. Green. 1998. Base-calling of automated sequencer traces using PHRED. I. Accuracy assessment. Genome Res. 8:175-185. [DOI] [PubMed] [Google Scholar]
- 24.Faulker, P., J. U. Kuzio, G. V. Williams, and J. A. Wilson. 1997. Analysis of p74, a PDV envelope protein of Autographa california nucleopolyhedrovirus required for occlusion body infectivity in vivo. J. Gen. Virol. 78:3091-3100. [DOI] [PubMed] [Google Scholar]
- 25.Gomi, S., K. Majima, and S. Maeda. 1999. Sequence analysis of the genome of Bombyx mori nucleopolyhedrovirus. J. Gen. Virol. 80:1323-1337. [DOI] [PubMed] [Google Scholar]
- 26.Granados, R. R., T. Nguyen, and B. Cato. 1978. An insect cell line persistently infected with a baculovirus-like particle. Intervirology 10:309-317. [DOI] [PubMed] [Google Scholar]
- 27.Guarino, L., and M. D. Summers. 1986. Interspersed homologous DNA of Autographa californica nuclear polyhedrosis virus enhances delayed-early gene expression. J. Virol. 60:215-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guttieri, M. C., and J. P. Burand. 1996. Nucleotide sequence, temporal expression, and transcriptional mapping of the p34 late gene of the Hz-1 insect virus. Virology 223:370-375. [DOI] [PubMed] [Google Scholar]
- 29.Guttieri, M. C., and J. P. Burand. 2001. Location, nucleotide sequence, and regulation of the p51 late gene of the Hz-1 insect virus: identification of a putative late regulatory element. Virus Genes 23:17-25. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto, Y., T. Hayakawa, Y. Ueno, T. Fugita, Y. Sano, and T. Matsumoto. 2000. Sequence analysis of the Plutella xylostella granulovirus genome. Virology 275:358-372. [DOI] [PubMed] [Google Scholar]
- 31.Hayakawa, T., G. F. Rohrmann, and Y. Hashimoto. 2000. Patterns of genome organization and content in lepidopteran baculoviruses. Virology 278:1-12. [DOI] [PubMed] [Google Scholar]
- 32.Hink, W. F. 1972. Insect tissue culture. Adv. Appl. Microb. 15:157-214. [DOI] [PubMed] [Google Scholar]
- 33.Hishinuma, F., and K. Hirai. 1991. Genome organization of the linear plasmid, pSKL, isolated from Saccharomyces kluyveri. Mol. Gen. Genet. 226:97-106. [DOI] [PubMed] [Google Scholar]
- 34.Huang, Y. S., M. Hedberg, and C. Y. Kawanish. 1982. Characterization of the DNA of a nonoccluded baculovirus, Hz-1 V. J. Virol. 43:174-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ignoffo, C. M., M. Shapior, and W. F. Hink. 1971. Replication and serial passage of infectious Heliothis nuclear polyhedrosis virus in an established line of Heliothis zea cells. J. Invertebr. Pathol. 18:131-134. [DOI] [PubMed] [Google Scholar]
- 36.Ijkel, W. F., E. A. van Strien, J. G. M. Jeldens, R. Broer, D. Zuiderma, R. W. Goldbach, and J. M. Vlak. 1999. Sequence and organization of the Spodoptera exigua multicapsid nucleopolyhedrovirus genome. J. Gen. Virol. 80:3289-3304. [DOI] [PubMed] [Google Scholar]
- 37.Ives, D. H., and S. Ikeda. 1998. Life on the salvage path: the deoxynucleoside kinase of Lactobacillus acidophilus R-26. Prog. Nucleic Acid Res. Mol. Biol. 59:205-255. [DOI] [PubMed] [Google Scholar]
- 38.Jordan, A., and P. Reichard. 1998. Ribonucleotide reductases. Annu. Rev. Biochem. 67:71-98. [DOI] [PubMed] [Google Scholar]
- 39.Kelly, D. C., T. Lescott, M. D. Ayres, D. Carey, A. Countts, and K. A. Harrap. 1981. Induction of a nonoccluded baculovirus persistently infecting Heliothis zea cells by Heliothis armigera and Trichopuisa ni nuclear polyhedrosis virus. Virology 112:174-180. [DOI] [PubMed] [Google Scholar]
- 40.Ko, R., K. Okano, and S. Maeda. 2000. Structural and functional analysis of the Xestia c-nigrum granulovirus matrix metalloproteinase. J. Virol. 74:11240-11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kool, M., and J. M. Vlak. 1993. The structural and functional organization of the Autographa californica nuclear polyhedrosis virus genome. Arch. Virol. 130:1-16. [DOI] [PubMed] [Google Scholar]
- 42.Korngberg, A., and T. A. Baker. 1992. DNA replication, 2nd ed. Freeman, San Francisco, Calif.
- 43.Kuzio, J., R. Jacques, and P. Faulkner. 1989. Identification of p74, a gene essential for virulence of baculovirus occlusion bodies. Virology 173:759-763. [DOI] [PubMed] [Google Scholar]
- 43a.Kuzio, J., M. N. Pearson, S. H. Harwood, C. J. Funk, J. T. Evans, J. M. Slavicek, and G. F. Rohrmann. 1999. Sequence and analysis of the genome of a baculovirus pathogenic Lymantria dispar. Virology 253:17-34. [DOI] [PubMed] [Google Scholar]
- 44.McIntosh, A. H., and C. M. Ignoffo. 1981. Establishment of a persistent baculovirus infection in a lepidopteran cell line. J. Invertebr. Pathol. 8:395-403. [Google Scholar]
- 45.McLachlin, J. R., and Miller, L. K. 2001. Identification and characterization of vlf-1, a baculovirus gene involved in very late gene expression. J. Virol. 68:7746-7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moser, B. A., J. J. Bencnel, S. E. White, C. Afonos, G. Kutish, S. Shanker, and E. Almira. 2001. Morphological and molecular evidence that Culex nigripalpus baculvoirus is an unusual member of the family Baculoviridae. J. Gen. Virol. 82:283-297. [DOI] [PubMed] [Google Scholar]
- 47.Muller, K., C. Tidona, A. U. Bahr, and G. Darai. 1998. Identification of a thymidylate synthase gene within the genome of Chilo iridescent virus. Virus Genes 17:243-258. [DOI] [PubMed] [Google Scholar]
- 48.Nicholas, J., V. Ruvolo, J. Zong, S. Ciufo, H. G. Guo, M. Reitz, and G. S. Hayward. 1997. A single 13-kilobase divergent locus in the Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genome contains nine open reading frames that are homologous to or related to cellular proteins. J. Virol. 71:1963-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pearson, M. N., and G. F. Rohrmann. 1995. Lymanthia dispar nuclear polyhedrosis virus homologous regions: characterization of their ability to function as replication origins. J. Virol. 69:213-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearson, W. R. 1990. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 183:63-98. [DOI] [PubMed] [Google Scholar]
- 51.Ralston, A. L., Y. S. Huang, and C. Y. Kawanish. 1981. Cell culture studies with the IMC-Hz-1 nonoccluded virus. Virology 115:33-44. [DOI] [PubMed] [Google Scholar]
- 52.Roe, R. M., and K. Venkatech. 1990. Metabolism of juvenile hormones: degradation and titer regulation, p. 125-180. In A. P. Gupta (ed.), Morphogenic hormones of arthropods, vol. 1. Rutgers University Press, New Brunswick, N.J.
- 53.Rossnick, M. J. 1997. Mechanisms of plant virus evolution. Annu. Rev. Phytopathol. 354:191-209. [DOI] [PubMed] [Google Scholar]
- 54.Rutherford, K., J. Parkhill, J. Crook, T. Horsnell, P. Rice, M.-A. Rajandream, and B. Barrell. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944-945. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu, N., and T. Gojobori. 2000. How can human and simian immunodeficiency virused utilize chemokine receptors as their coreceptors? Gene 259:199-205. [DOI] [PubMed] [Google Scholar]
- 56.Slack, J. M., E. M. Pougherty, and S. D. Lawrence. 2001. A study of the Autographa californica multiple nucleopolyhedrosis virus ODV envelope protein p74 using a GFP tag. J. Gen. Virol. 82:2279-2287. [DOI] [PubMed] [Google Scholar]
- 57.Smith, G. E., and Summers, M. D. 1982. DNA homology among subgroup A, B, and C baculovirus. Virology 123:393.. [DOI] [PubMed] [Google Scholar]
- 58.Summers, M. D., and G. E. Smith. 1987. A manual of methods for baculovirus vectors and insect cell culture procedure, p. 33-35. Department of Entomology Texas Agricultural Experiment Station, Texas A&M University, College Station, Tex.
- 59.Volkman, L. E., G. W. Blissard, P. D. Friesen, B. A. Keddie, R. D. Possee, and D. A. Theilmann. 1995. Virus taxonomy: The classification and nomenclature of viruses, p. 104-113. In F. A. Murphy, C. M. Fauquet, D. H. L. Bishop, S. A. Ghabrial, A. W. Javis, G. P. Martelli, M. A. Mayo, and M. D. Summers (ed.), The sixth report of the International Committee on Taxonomy of Viruses. Springer-Verlag Wein, Inc., New York, N.Y.
- 60.Yang, S., and L. K. Miller. 1998. Expression and mutational analysis of the baculovirus very late factor 1 (vlf-1) gene. Virology 245:99-109. [DOI] [PubMed] [Google Scholar]