Abstract
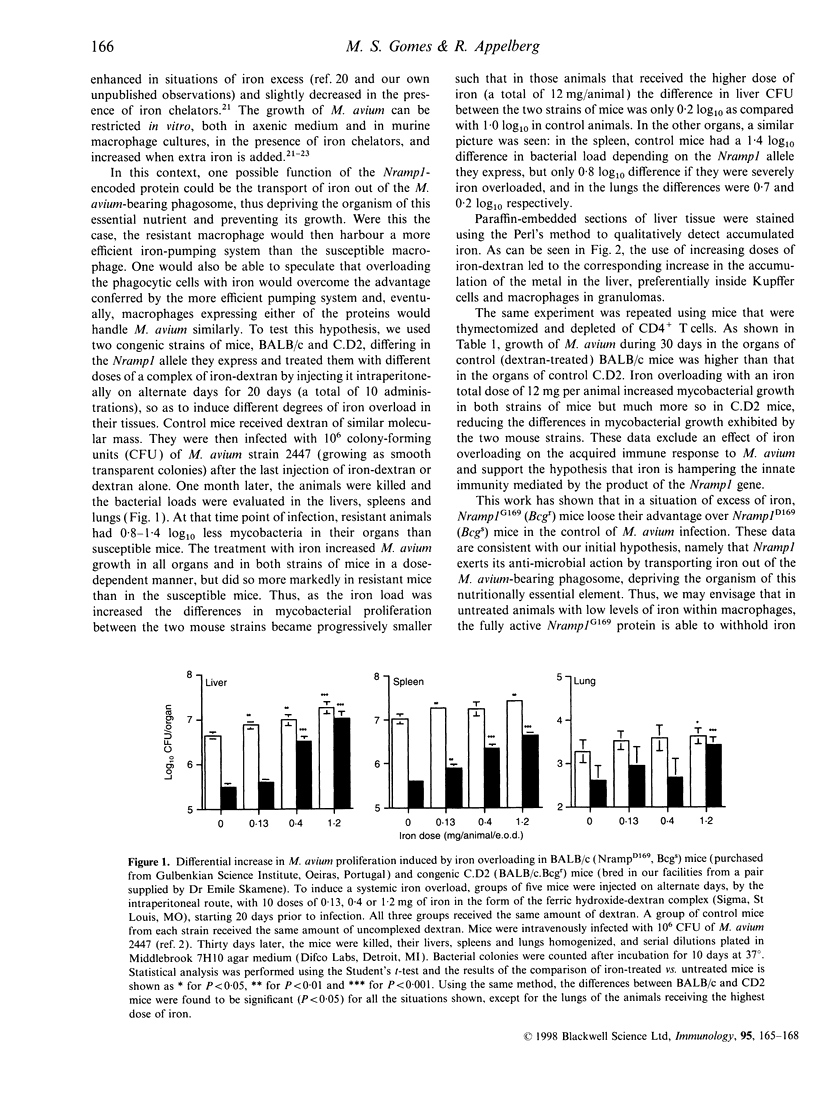
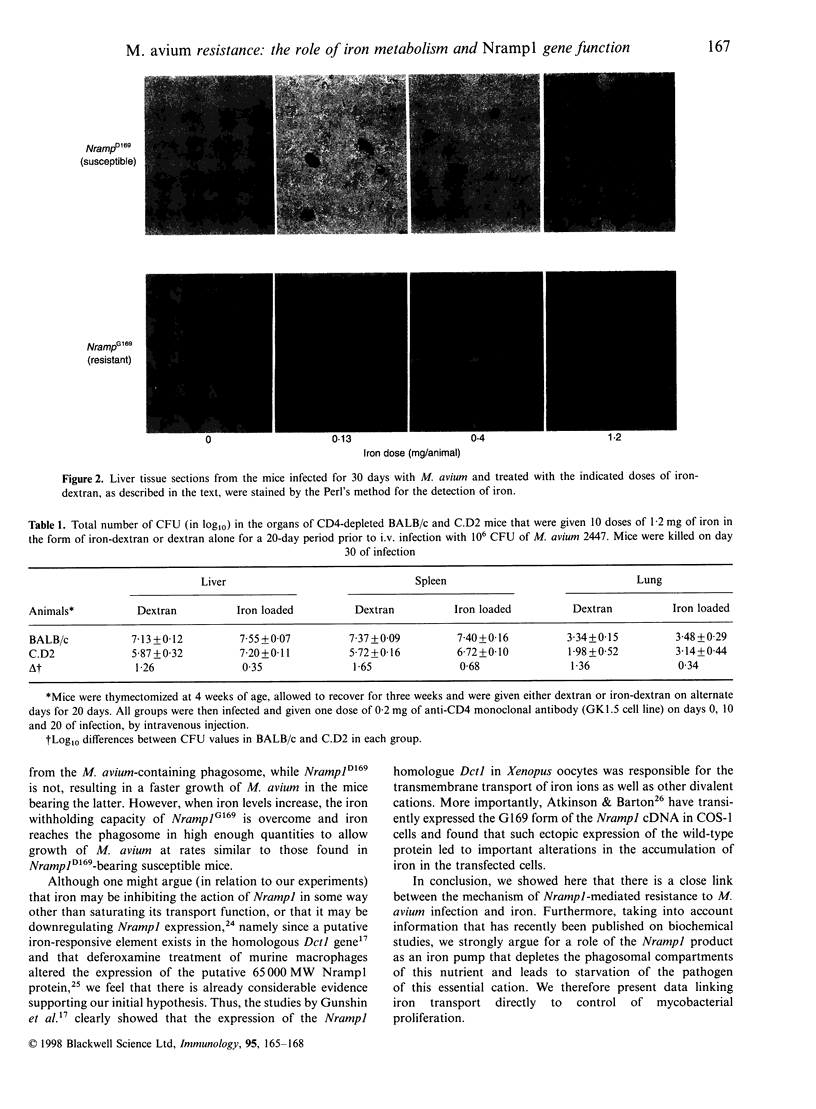
In the mouse, the progression of the Mycobacterium avium infection is highly dependent on the Nramp1 gene. Strains of mice that express the Nramp1D169 allele are highly susceptible to M. avium infections, while Nramp1G169 strains of mice can control them. Recently, the Nramp1 gene has been cloned and characterized as coding a transmembrane protein, probably involved in divalent cation transport. One possible function of this protein could be the transport of iron out of the parasite-harbouring phagosome. Previous work in our lab has shown both in vitro (in macrophage cultures) and in vivo, that the growth rate of M. avium is highly dependent on the amount of iron available in the system. To try to correlate this with the Nramp1 gene function, BALB/c (susceptible) and C.D2 (resistant) congenic mice were treated for 20 days with different doses of iron-dextran, so as to induce different degrees of iron overload, and infected with M. avium 2447. Iron administration increased M. avium growth in infected organs in a dose-dependent manner at the same time as it decreased the difference in mycobacterial growth between the two mouse strains. These results indicate that an excess of iron hampers Nramp1-encoded function, strongly suggesting a direct involvement of the Nramp1-encoded protein in the transport of this cation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg R., Sarmento A. M. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin Exp Immunol. 1990 Jun;80(3):324–331. doi: 10.1111/j.1365-2249.1990.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson P. G., Barton C. H. Ectopic expression of Nramp1 in COS-1 cells modulates iron accumulation. FEBS Lett. 1998 Mar 27;425(2):239–242. doi: 10.1016/s0014-5793(98)00236-1. [DOI] [PubMed] [Google Scholar]
- Atkinson P. G., Blackwell J. M., Barton C. H. Nramp1 locus encodes a 65 kDa interferon-gamma-inducible protein in murine macrophages. Biochem J. 1997 Aug 1;325(Pt 3):779–786. doi: 10.1042/bj3250779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton C. H., White J. K., Roach T. I., Blackwell J. M. NH2-terminal sequence of macrophage-expressed natural resistance-associated macrophage protein (Nramp) encodes a proline/serine-rich putative Src homology 3-binding domain. J Exp Med. 1994 May 1;179(5):1683–1687. doi: 10.1084/jem.179.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy R., Ruwende C., Corrah T., McAdam K. P., Whittle H. C., Hill A. V. Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. N Engl J Med. 1998 Mar 5;338(10):640–644. doi: 10.1056/NEJM199803053381002. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M., Barton C. H., White J. K., Roach T. I., Shaw M. A., Whitehead S. H., Mock B. A., Searle S., Williams H., Baker A. M. Genetic regulation of leishmanial and mycobacterial infections: the Lsh/Ity/Bcg gene story continues. Immunol Lett. 1994 Dec;43(1-2):99–107. doi: 10.1016/0165-2478(94)00161-8. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M., Black G. F., Peacock C. S., Miller E. N., Sibthorpe D., Gnananandha D., Shaw J. J., Silveira F., Lins-Lainson Z., Ramos F. Immunogenetics of leishmanial and mycobacterial infections: the Belem Family Study. Philos Trans R Soc Lond B Biol Sci. 1997 Sep 29;352(1359):1331–1345. doi: 10.1098/rstb.1997.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. M. Genetics of host resistance and susceptibility to intramacrophage pathogens: a study of multicase families of tuberculosis, leprosy and leishmaniasis in north-eastern Brazil. Int J Parasitol. 1998 Jan;28(1):21–28. doi: 10.1016/s0020-7519(97)00175-6. [DOI] [PubMed] [Google Scholar]
- Castro A. G., Minóprio P., Appelberg R. The relative impact of bacterial virulence and host genetic background on cytokine expression during Mycobacterium avium infection of mice. Immunology. 1995 Aug;85(4):556–561. [PMC free article] [PubMed] [Google Scholar]
- Dhople A. M., Dhople A. A., Ibanez M. A. In vitro activities of 2,2'-bipyridyl analogues against Mycobacterium avium and M. tuberculosis. Tuber Lung Dis. 1995 Apr;76(2):136–140. doi: 10.1016/0962-8479(95)90556-1. [DOI] [PubMed] [Google Scholar]
- Dhople A. M., Ibanez M. A., Poirier T. C. Role of iron in the pathogenesis of Mycobacterium avium infection in mice. Microbios. 1996;87(351):77–87. [PubMed] [Google Scholar]
- Fleming M. D., Romano M. A., Su M. A., Garrick L. M., Garrick M. D., Andrews N. C. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A. 1998 Feb 3;95(3):1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming M. D., Trenor C. C., 3rd, Su M. A., Foernzler D., Beier D. R., Dietrich W. F., Andrews N. C. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997 Aug;16(4):383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- Govoni G., Gauthier S., Billia F., Iscove N. N., Gros P. Cell-specific and inducible Nramp1 gene expression in mouse macrophages in vitro and in vivo. J Leukoc Biol. 1997 Aug;62(2):277–286. doi: 10.1002/jlb.62.2.277. [DOI] [PubMed] [Google Scholar]
- Gruenheid S., Pinner E., Desjardins M., Gros P. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J Exp Med. 1997 Feb 17;185(4):717–730. doi: 10.1084/jem.185.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H., Mackenzie B., Berger U. V., Gunshin Y., Romero M. F., Boron W. F., Nussberger S., Gollan J. L., Hediger M. A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997 Jul 31;388(6641):482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Huang J. H., Oefner P. J., Adi V., Ratnam K., Ruoss S. J., Trako E., Kao P. N. Analyses of the NRAMP1 and IFN-gammaR1 genes in women with Mycobacterium avium-intracellulare pulmonary disease. Am J Respir Crit Care Med. 1998 Feb;157(2):377–381. doi: 10.1164/ajrccm.157.2.9706012. [DOI] [PubMed] [Google Scholar]
- Skamene E. The Bcg gene story. Immunobiology. 1994 Oct;191(4-5):451–460. doi: 10.1016/S0171-2985(11)80451-1. [DOI] [PubMed] [Google Scholar]
- Supek F., Supekova L., Nelson H., Nelson N. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc Natl Acad Sci U S A. 1996 May 14;93(10):5105–5110. doi: 10.1073/pnas.93.10.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S. M., Malo D., Vogan K., Skamene E., Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993 May 7;73(3):469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Vidal S. M., Pinner E., Lepage P., Gauthier S., Gros P. Natural resistance to intracellular infections: Nramp1 encodes a membrane phosphoglycoprotein absent in macrophages from susceptible (Nramp1 D169) mouse strains. J Immunol. 1996 Oct 15;157(8):3559–3568. [PubMed] [Google Scholar]
- Vidal S., Tremblay M. L., Govoni G., Gauthier S., Sebastiani G., Malo D., Skamene E., Olivier M., Jothy S., Gros P. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med. 1995 Sep 1;182(3):655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]



