Abstract
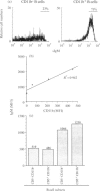
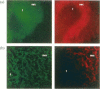
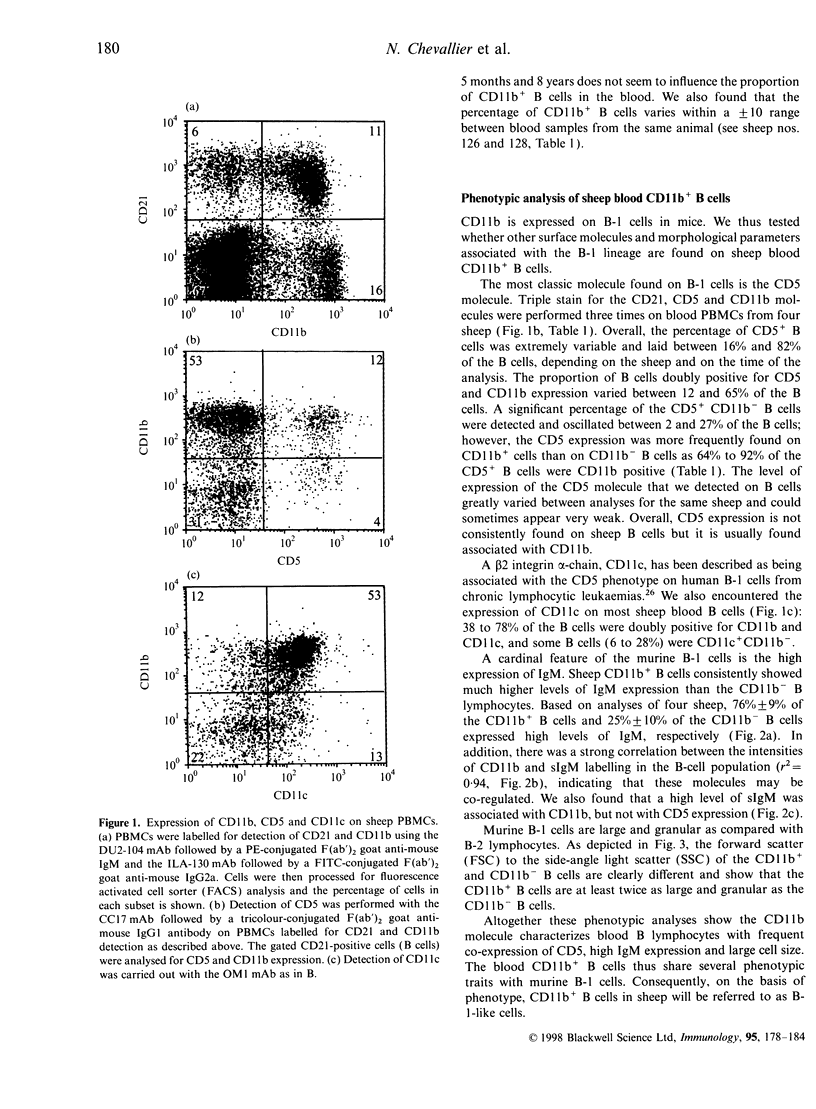
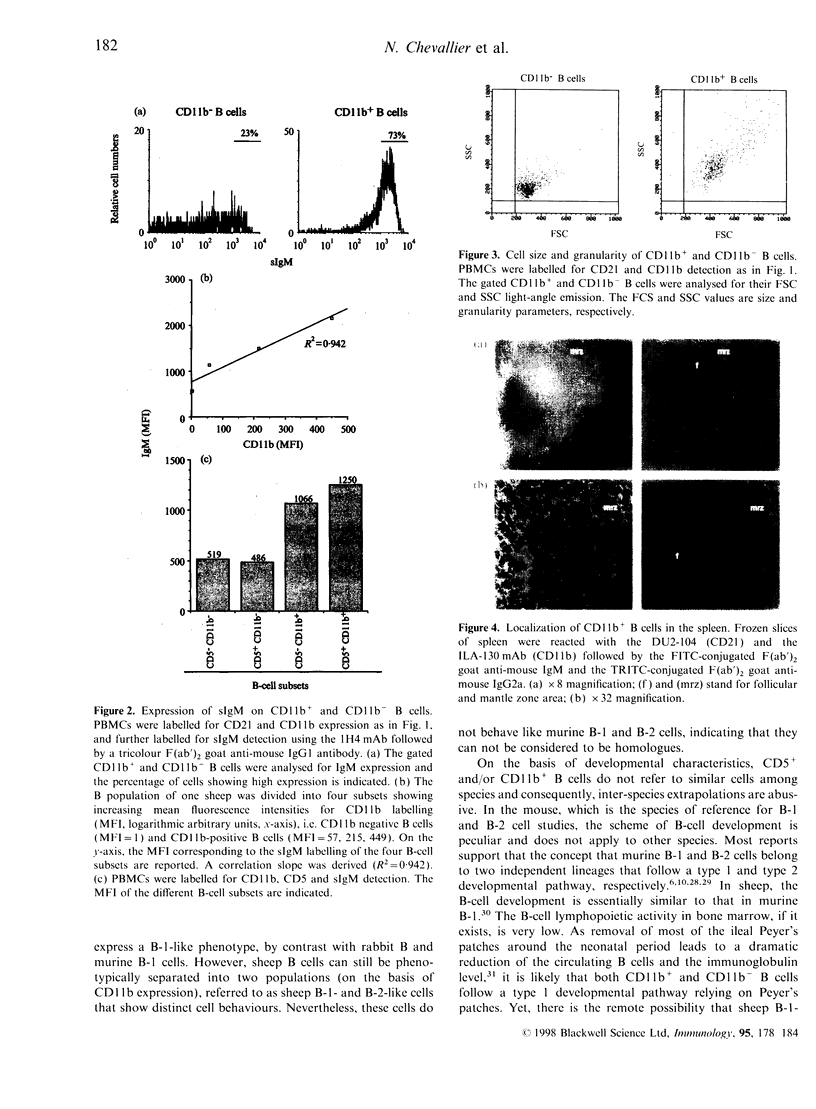
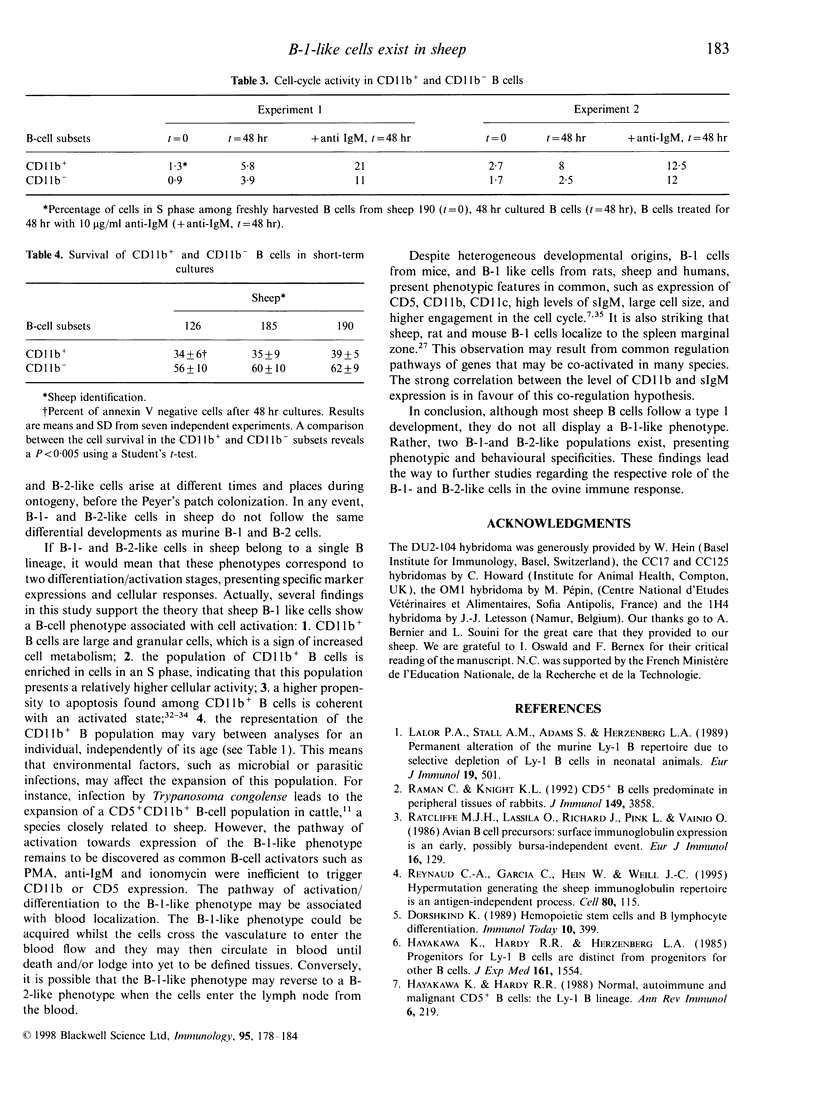
Two populations of B lymphocytes, B-1 (CD5+ and/or CD11b+) and B-2 (CD5- and CD11b-) cells have been described. In mice, which is the species of reference for B-1 and B-2 cell studies, these two subsets present different developmental schemes, phenotypes, antibody repertoires, localization and behaviours. Interestingly, in sheep, B cells rearrange their immunoglobulin (Ig) loci around the neonatal period, similarly to murine B-1 cells. However, the phenotype of the sheep B cells has not been characterized with regards to their developmental pathway. In this report, we show that two sheep B-cell subsets can be distinguished on the basis of CD11b expression. Relative to CD11b- B cells, the CD11b+ B cells frequently co-express CD5, CD11c, higher levels of surface IgM (sIgM), show larger cell size and higher cell-cycling activity, and thus present a B-1-like phenotype. However, unlike murine B-1 cells, sheep B-1 like cells mainly localize in blood, display a higher propensity to spontaneous apoptosis relative to B-2-like cells, and proliferate after sIgM stimulation. Our data show that despite neonatal immunoglobulin loci rearrangements, sheep B cells do not all express a B-1-like phenotype. However, B-1-and B-2-like cells co-exist and present phenotypic and behavioural specificities. Nevertheless, sheep B-1-and B-2-like cells differ from the murine B-1 and B-2 cells in their cell behaviour. These subsets can thus not be considered as true homologues among species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazin H., Gray D., Platteau B., MacLennan I. C. Distinct delta + and delta - B-lymphocyte lineages in the rat. Ann N Y Acad Sci. 1982;399:157–174. doi: 10.1111/j.1749-6632.1982.tb25671.x. [DOI] [PubMed] [Google Scholar]
- Bikah G., Carey J., Ciallella J. R., Tarakhovsky A., Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science. 1996 Dec 13;274(5294):1906–1909. doi: 10.1126/science.274.5294.1906. [DOI] [PubMed] [Google Scholar]
- Birkebak T. A., Palmer G. H., Davis W. C., McElwain T. F. Quantitative characterization of the CD5 bearing lymphocyte population in the peripheral blood of normal sheep. Vet Immunol Immunopathol. 1994 May;41(1-2):181–186. doi: 10.1016/0165-2427(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Braun J. Spontaneous in vitro occurrence and long-term culture of murine B lymphoblast cell lines. J Immunol. 1983 May;130(5):2113–2116. [PubMed] [Google Scholar]
- Brugnoni D., Airò P., Facchetti F., Blanzuoli L., Ugazio A. G., Cattaneo R., Notarangelo L. D. In vitro cell death of activated lymphocytes in Omenn's syndrome. Eur J Immunol. 1997 Nov;27(11):2765–2773. doi: 10.1002/eji.1830271104. [DOI] [PubMed] [Google Scholar]
- Chevallier N., Berthelemy M., Le Rhun D., Lainé V., Levy D., Schwartz-Cornil I. Bovine leukemia virus-induced lymphocytosis and increased cell survival mainly involve the CD11b+ B-lymphocyte subset in sheep. J Virol. 1998 May;72(5):4413–4420. doi: 10.1128/jvi.72.5.4413-4420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil I., Delon P., Parodi A. L., Levy D. T-B cell cooperation for bovine leukemia virus expression in ovine lymphocytes. Leukemia. 1988 May;2(5):313–317. [PubMed] [Google Scholar]
- De la Hera A., Alvarez-Mon M., Sanchez-Madrid F., Martinez C., Durantez A. Co-expression of Mac-1 and p150,95 on CD5+ B cells. Structural and functional characterization in a human chronic lymphocytic leukemia. Eur J Immunol. 1988 Jul;18(7):1131–1134. doi: 10.1002/eji.1830180725. [DOI] [PubMed] [Google Scholar]
- Dorshkind K. Hemopoietic stem cells and B-lymphocyte differentiation. Immunol Today. 1989 Dec;10(12):399–401. doi: 10.1016/0167-5699(89)90033-9. [DOI] [PubMed] [Google Scholar]
- Gerber H. A., Morris B., Trevella W. The role of gut-associated lymphoid tissues in the generation of immunoglobulin-bearing lymphocytes in sheep. Aust J Exp Biol Med Sci. 1986 Jun;64(Pt 3):201–213. doi: 10.1038/icb.1986.22. [DOI] [PubMed] [Google Scholar]
- Gerber H. A., Morris B., Trevella W. The role of gut-associated lymphoid tissues in the generation of immunoglobulin-bearing lymphocytes in sheep. Aust J Exp Biol Med Sci. 1986 Jun;64(Pt 3):201–213. doi: 10.1038/icb.1986.22. [DOI] [PubMed] [Google Scholar]
- Gougeon M. L., Lecoeur H., Dulioust A., Enouf M. G., Crouvoiser M., Goujard C., Debord T., Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996 May 1;156(9):3509–3520. [PubMed] [Google Scholar]
- Gupta V. K., McConnell I., Pepin M., Davis W. C., Dalziel R. G., Hopkins J. Biochemical and phenotypic characterization of the ovine beta 2 (leucocyte) integrins. J Comp Pathol. 1995 May;112(4):339–349. doi: 10.1016/s0021-9975(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Stall A. M., Herzenberg L. A., Herzenberg L. A. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur J Immunol. 1986 Oct;16(10):1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Kantor A. B. B-cell lineages exist in the mouse. Immunol Today. 1993 Feb;14(2):79–90. doi: 10.1016/0167-5699(93)90063-Q. [DOI] [PubMed] [Google Scholar]
- Kantor A. B. The development and repertoire of B-1 cells (CD5 B cells). Immunol Today. 1991 Nov;12(11):389–391. doi: 10.1016/0167-5699(91)90136-H. [DOI] [PubMed] [Google Scholar]
- Kipps T. J. The CD5 B cell. Adv Immunol. 1989;47:117–185. doi: 10.1016/s0065-2776(08)60663-x. [DOI] [PubMed] [Google Scholar]
- Lalor P. A., Stall A. M., Adams S., Herzenberg L. A. Permanent alteration of the murine Ly-1 B repertoire due to selective depletion of Ly-1 B cells in neonatal animals. Eur J Immunol. 1989 Mar;19(3):501–506. doi: 10.1002/eji.1830190314. [DOI] [PubMed] [Google Scholar]
- Letesson J. J., Mager A., Mammerickx M., Burny A., Depelchin A. B cells from bovine leukemia virus- (BLV) infected sheep with hematological disorders express the CD5 T cell marker. Leukemia. 1990 May;4(5):377–379. [PubMed] [Google Scholar]
- Naessens J. Surface Ig on B lymphocytes from cattle and sheep. Int Immunol. 1997 Mar;9(3):349–354. doi: 10.1093/intimm/9.3.349. [DOI] [PubMed] [Google Scholar]
- Naessens J., Williams D. J. Characterization and measurement of CD5+ B cells in normal and Trypanosoma congolense-infected cattle. Eur J Immunol. 1992 Jul;22(7):1713–1718. doi: 10.1002/eji.1830220708. [DOI] [PubMed] [Google Scholar]
- Oberg H. H., Lengl-Janssen B., Kabelitz D., Janssen O. Activation-induced T cell death: resistance or susceptibility correlate with cell surface fas ligand expression and T helper phenotype. Cell Immunol. 1997 Oct 10;181(1):93–100. doi: 10.1006/cimm.1997.1200. [DOI] [PubMed] [Google Scholar]
- Peters L. C., Brandhorst J. S., Hanna M. G., Jr Preparation of immunotherapeutic autologous tumor cell vaccines from solid tumors. Cancer Res. 1979 Apr;39(4):1353–1360. [PubMed] [Google Scholar]
- Pospisil R., Young-Cooper G. O., Mage R. G. Preferential expansion and survival of B lymphocytes based on VH framework 1 and framework 3 expression: "positive" selection in appendix of normal and VH-mutant rabbits. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6961–6965. doi: 10.1073/pnas.92.15.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press C. M., Hein W. R., Landsverk T. Ontogeny of leucocyte populations in the spleen of fetal lambs with emphasis on the early prominence of B cells. Immunology. 1993 Dec;80(4):598–604. [PMC free article] [PubMed] [Google Scholar]
- Proceedings of the 3rd workshop on ruminant leukocyte antigens. Davis, California, 16 July 1995. Vet Immunol Immunopathol. 1996 Aug;52(4):213–468. [PubMed] [Google Scholar]
- Pépin M., Cannella D., Fontaine J. J., Pittet J. C., Le Pape A. Ovine mononuclear phagocytes in situ: identification by monoclonal antibodies and involvement in experimental pyogranulomas. J Leukoc Biol. 1992 Feb;51(2):188–198. doi: 10.1002/jlb.51.2.188. [DOI] [PubMed] [Google Scholar]
- Raman C., Knight K. L. CD5+ B cells predominate in peripheral tissues of rabbit. J Immunol. 1992 Dec 15;149(12):3858–3864. [PubMed] [Google Scholar]
- Ratcliffe M. J., Lassila O., Pink J. R., Vainio O. Avian B cell precursors: surface immunoglobulin expression is an early, possibly bursa-independent event. Eur J Immunol. 1986 Feb;16(2):129–133. doi: 10.1002/eji.1830160204. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Garcia C., Hein W. R., Weill J. C. Hypermutation generating the sheep immunoglobulin repertoire is an antigen-independent process. Cell. 1995 Jan 13;80(1):115–125. doi: 10.1016/0092-8674(95)90456-5. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Mackay C. R., Müller R. G., Weill J. C. Somatic generation of diversity in a mammalian primary lymphoid organ: the sheep ileal Peyer's patches. Cell. 1991 Mar 8;64(5):995–1005. doi: 10.1016/0092-8674(91)90323-q. [DOI] [PubMed] [Google Scholar]
- Schwartz-Cornil I., Chevallier N., Belloc C., Le Rhun D., Lainé V., Berthelemy M., Mateo A., Levy D. Bovine leukaemia virus-induced lymphocytosis in sheep is associated with reduction of spontaneous B cell apoptosis. J Gen Virol. 1997 Jan;78(Pt 1):153–162. doi: 10.1099/0022-1317-78-1-153. [DOI] [PubMed] [Google Scholar]
- Sibinga N. E., Goldstein A. Opioid peptides and opioid receptors in cells of the immune system. Annu Rev Immunol. 1988;6:219–249. doi: 10.1146/annurev.iy.06.040188.001251. [DOI] [PubMed] [Google Scholar]