Abstract
Neuronal apoptosis within the central nervous system (CNS) is a characteristic feature of AIDS dementia, and it represents a common mechanism of neuronal death induced by neurotoxins (e.g., glutamate) released from human immunodeficiency virus (HIV)-infected macrophages (HIV/macrophage-induced neurotoxicity). Neuronal apoptosis may result from activation of the intrinsic (mitochondrial/bcl-2 regulated) or extrinsic (death receptor) pathways, although which pathway predominates in CNS HIV infection is unknown. Apoptosis initiated by the intrinsic pathway is typically blocked by antiapoptosis Bcl-2 family proteins, such as Bcl-2 and Bcl-xL, but whether these can block HIV/macrophage-induced neuronal apoptosis is unknown. To determine the potential role of the Bcl-2 family in HIV/macrophage-induced neuronal apoptosis, we developed a unique in vitro model, utilizing the NT2 neuronal cell line, primary astrocytes and macrophages, and primary CNS HIV type 1 (HIV-1) isolates. We validated our model by demonstrating that NT2.N neurons are protected against HIV-infected macrophages by N-methyl-d-aspartate (NMDA) glutamate receptor antagonists, similar to effects seen in primary neurons. We then established stable NT2.N neuronal lines that overexpress Bcl-2 or Bcl-xL (NT2.N/bcl-2 and NT2.N/bcl-xL, respectively) and determined their sensitivity to macrophages infected with primary R5, X4, and R5/X4 HIV-1 isolates. We found that NT2.N/bcl-2 and NT2.N/bcl-xL neurons were resistant to apoptosis induced by either R5, X4, or R5/X4 isolates and that resistance was abrogated by a Bcl-2 antagonist. Thus, the NMDA receptor/bcl-2-regulated apoptotic pathway contributes significantly to HIV/macrophage-induced neuronal apoptosis, and Bcl-2 family proteins protect neurons against the spectrum of primary HIV-1 isolates. Modulation of bcl-2 gene expression may therefore offer adjunctive neuroprotection against development of AIDS dementia.
Neurodegeneration is a characteristic feature of AIDS dementia and is commonly associated with neuronal apoptosis in the brain in both pediatric and adult patients (1, 3, 30, 43, 53, 59). Clinical studies suggest that neuronal loss is a chronic, progressive process that manifests symptomatically years after seroconversion, and in vitro evidence supports a role for glutamate, the human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein, Tat, Vpr, proinflammatory cytokines, nitric oxide, and other cellular factors released by HIV-1-infected macrophages (HIV/macrophage-induced neurotoxicity). In vitro evidence suggests that each of these factors can induce toxicity either directly or indirectly through downstream effects at the N-methyl-d-aspartate (NMDA)-type glutamate receptor (reviewed in references 27, 29, and 35). Some apoptotic effects of HIV proteins may be mediated via chemokine receptors (21, 72).
There are two well-described pathways for induction of apoptosis: the extrinsic, or death receptor-mediated (tumor necrosis factor alpha and Fas); and the intrinsic, or mitochondrial mediated (14, 57, 61). Both pathways ultimately converge via activation of downstream effector caspases, and in general, the intrinsic pathway is blocked by members of the Bcl-2 family of proteins (Bcl-2 and Bcl-xL) (61). The Bcl-2 family is involved in modulating neuronal apoptosis by a variety of brain insults (12, 32, 55), but its role in HIV-1-induced neuronal apoptosis remains unknown. Members of the Bcl-2 protein family include the proapoptosis protein Bax-α and the antiapotosis proteins Bcl-2 and Bcl-xL (reviewed in references 41 and 6, respectively). Induction of Bax-α expression in neurons is associated with apoptosis (4, 9, 12, 31, 32, 40, 63, 67). In contrast, induction of Bcl-2 or Bcl-xL expression may prevent neuronal apoptosis induced by various mitochondrial-mediated insults, including NMDA receptor overactivation (2, 7, 60, 68). In some cell types, induction of mitochondrial damage through activation of the extrinsic pathway may occur through cleavage of Bid, another proapoptotic Bcl-2 family member (34, 39).
Notably, immunohistochemical analyses of HIV-1-infected brain have shown that infection of macrophages/microglia is clearly associated with apoptosis in neurons as well as in macrophages/microglia (1, 24, 30, 59). A possible link between HIV/macrophage-induced apoptosis and bcl-2 gene family expression was suggested by Krajewski et al. (30). These investigators demonstrated increased Bax-α expression in both HIV-infected and noninfected apoptotic macrophages/microglia in human brain, although Bax-α expression was not detected in apoptotic neurons. Interestingly, no differences were seen in neuronal expression of Bcl-2 or Bcl-xL between HIV-1-infected brain and noninfected brain. This suggests that failure of induction of Bcl-2 or Bcl-xL expression in subsets of neurons in HIV-infected brain may render them vulnerable to apoptosis-inducing effects of HIV-1.
To better understand the mechanisms of HIV-1-induced neuronal apoptosis and to define the role of the Bcl-2 family in modulating neuronal cell responses to HIV-1 apoptosis signals, we examined the effects of neuronal Bcl-2 and Bcl-xL expression on the susceptibility of human neurons to HIV-induced apoptosis. To do this, we developed a unique HIV/macrophage neuronal apoptosis model utilizing NT2.N human neurons, primary astrocytes, and monocyte-derived macrophages, as well as primary central nervous system (CNS) HIV-1 isolates. We demonstrated that NMDA glutamate receptor antagonists block HIV/macrophage-induced NT2.N apoptosis, similar to blocking effects against gp120 previously demonstrated in primary fetal mixed neuronal-glial cell cultures exposed to NMDA receptor antagonists (19, 33, 36). We then exploited our ability to transfect NT2 cells to establish stably transfected Bcl-2- and Bcl-xL-expressing lines (NT2.N/bcl-2 and NT2.N/bcl-xL, respectively) and compared the ability of HIV-1-infected macrophages to induce apoptosis in native NT2.N neurons as well as NT2.N/bcl-2 and NT2.N/bcl-xL neurons. We found that (i) primary HIV-1 strains of the R5, X4, and R5/X4 phenotypes induce neuronal apoptosis mediated by neuronal NMDA receptors, and they vary in their ability to do so; (ii) HIV/monocyte-derived macrophage (MDM)-induced neuronal apoptosis may occur despite endogenous basal Bcl-2 and Bcl-xL expression; and (iii) modest overexpression of either Bcl-2 or Bcl-xL in neurons can block HIV/macrophage-induced neuronal apoptosis. This is the first demonstration of a protective effect of Bcl-2 and/or Bcl-xL against HIV-1-induced neuronal apoptosis and suggests that the intrinsic mitochondrial-associated apoptosis pathway is the major pathway of neuronal death induced by HIV-infected macrophages. Modulation of the intrinsic apoptosis pathway from the level of surface receptor blockade through downstream targets regulated by the bcl-2 gene family of proteins may offer additional targets for neuroprotective strategies against HIV-1.
MATERIALS AND METHODS
Cell culture.
Undifferentiated human teratocarcinoma cells, NTera 2/c1.D1 (NT−), were differentiated as previously described (54). Briefly, 2.7 × 106 cells were seeded in a 75-cm2 flask and exposed to 10 μM retinoic acid for 5 weeks. The cells were then replated onto nine tissue culture dishes (10 cm in diameter), and 7 days later, the neurons were trypsin separated from nonneuronal background cells and mechanically dispersed into a single-cell suspension for final replating. For use in Western blot experiments, neurons were replated onto plastic wells coated with Matrigel (Collaborative Biomedical Products, Bedford, Mass.) in Dulbecco's modified Eagle's medium with 10% fetal bovine serum (FBS), 100 U of penicillin per ml, 100 U of streptomycin per ml, 1 μM cytosine arabinoside, 10 μM fluorodeoxyuridine, and 10 μM uridine (Sigma) at a density of 3 × 104 cells per cm2. The differentiated neurons (NT2.N) were harvested 4 to 6 weeks after this final plating. For terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay protocols, the neurons were plated onto glass coverslips (2 × 105 cells per cm2) containing a feeder layer of rat astrocytes, which were prepared from rat embryos as previously described (58). Astrocytes were harvested from animals under protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Coverslips were maintained for 5 to 7 weeks before use, to ensure full expression of NMDA- and non-NMDA-type glutamate receptors (56).
Establishment of NT2/bcl-2 and NT2/bcl-xL cell lines.
The pSFFV-NEO-Bcl-2 and pCDFLAGBcl-xL plasmids were generously provided by S. J. Korsmeyer (22) and Jonathan Ham (63), respectively. The plasmids were transfected into NTera 2 cells (10 μg of DNA per 3 × 105 cells) by using a calcium phosphate transfection kit (5′prime →3′ prime, Inc.). NT2/bcl-2 cells were produced by transfection of pSFFV-NEO-Bcl-2 (10 μg of DNA per 3 × 105 cells) followed by G418 (Geneticin; Gibco BRL) selection (300 μg/ml) for 2 months. NT2/bcl-xL cells were similarly produced by cotransfection of pSV2-NEO and pCDFLAGBcl-xL. Protein expression was confirmed by Western blotting.
Western blot analysis.
Cell cultures were washed with phosphate-buffered saline (PBS) and then lysed with radioimmunoprecipitation assay lysis buffer containing a proteinase inhibitor cocktail (Sigma). Equivalent amounts (25 μg) of total cell lysate protein aliquot were loaded onto a 12% polyacrylamide gel and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gel was blotted onto an Immobilon P membrane (Millipore, Bedford, Mass.), and the filters were blocked in Tris-buffered saline (TBS)-0.05% Tween 20 (TBS-T) and 5% milk, followed by incubation with either anti-human Bcl-2 (Pharmingen), -Bcl-xL, -Bax, or -actin antibodies (Santa Cruz). All antibodies were diluted in TBS-T to a final concentration of 2 μg/ml. The membrane was probed with primary antibody for 2 h at room temperature with gentle rocking, washed five times for 15 min each with TBS-T, and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Amersham) at a 1:1,000 dilution in TBS-T for 1 h at room temperature. After being washed five times for 15 min each, the membranes were developed with an ECL enhanced chemiluminescence Western blot kit (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.) and exposed to Hyperfilm (Amersham) at room temperature. The films were scanned for densitometric analysis with Personal Densitometer SI and ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.). Standard curves were generated to ensure linearity of signal intensity over the range of protein amounts loaded into gel lanes (6 to 50 μg of total protein per lane; r2 ≥ 0.99). Comparisons of densitometric signal intensities for Bcl-2, Bcl-xL, Bax-α, and actin were made only within this linearity range.
HIV-1 isolates.
The peripheral blood isolate 89.6 is a dualtropic R5/X4 prototype primary isolate that has been extensively characterized for tropism and chemokine receptor utilization (69). The doge, jago, and tybe isolates were derived from cell-free cerebrospinal fluid (CSF) harvested by lumbar puncture from patients either undergoing either diagnostic testing or participating in clinical research trials at the University of Pennsylvania. CSF was cocultured with phytohemagglutinin (PHA) or interleukin-2 (IL-2)-stimulated peripheral blood mononuclear cells (PBMC) from seronegative volunteers, and cell-free supernatants were analyzed for infectious virus by infection of fresh PBMC and MDM. Chemokine receptor use was determined by ability to infect HOS-CD4 cell lines expressing either CXCR4 or CCR5 chemokine receptors (not shown). All HIV-1 stocks were prepared by infection of primary peripheral blood lymphcytes (PBL), assayed for p24gag antigen content, and verified to be mycoplasma free by PCR analysis (ATCC Mycoplasma detection kit, and Stratagene Mycoplasma Plus PCR primer set).
MDM were isolated from PBMC of healthy volunteers as previously described (8). Cells were harvested according to protocols approved by the University of Pennsylvania Committee on Studies Involving Human Beings. Cells were cultured (1.5 × 105 cells per cm2) in the presence of macrophage-colony stimulating factor (M-CSF; 100 U/ml) in macrophage media for 7 days, inoculated with cell-free HIV-1 inoculum, and then monitored for supernatant p24 antigen by enzyme-linked immunosorbent assay (ELISA) (NEN Life Science Products, Inc.). All infections were performed with equivalent amounts of viral inocula (70 ng/106 cells), as determined by p24 antigen content. In control cultures, zidovudine (AZT; 1 μM pretreatment for 60 min) was used to block HIV replication. Culture supernatants were collected at selected time points and stored at −80°C.
Induction and quantitation of neuronal apoptosis.
Neurons were cultured on glass coverslips on a feeder layer of rodent astrocytes at a density of 5 × 104 cells per cm2. After 5 to 7 weeks, HIV-1-infected MDM culture supernatants were added to a final dilution of 1:4. Control coverslips were exposed to either conditioned medium from noninfected MDM or HIV-1-infected MDM with and without AZT (1 μM) or glutamate (1 mM). Forty-eight hours after addition of conditioned medium, cultures were fixed in 3.7% formaldehyde as subjected to TUNEL assay with a TdT-FragEL DNA fragmentation detection kit (Oncogene Research Products, Cambridge, Mass.) according to the manufacturer's protocol by using diaminobenzidine (DAB) substrate. All coverslips were stained with Hoechst 33342 (Calbiochem) to assess nuclear morphology. Selected coverslips were stained with anti-microtubule-associated protein-2 (MAP-2) antibody to identify neurons, and with an anti-glial fibrillary acidic protein (GFAP) antibody to identify astrocytes (58). For antibody labeling, coverslips were fixed in 4% paraformaldehyde-PBS-4% sucrose for 20 min at 4°C and then permeabilized in methanol (10 min at 4°C) followed by 0.2% Triton X-100 (10 min at 4°C). Blocking was performed with 10% FBS-PBS for 30 min at room temperature. Antibody was reconstituted to a final concentration of 1 μg/ml in 10% FBS-PBS and incubated with cells for 1 h at room temperature. Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse secondary antibody was used for detection. For controls, isotype-matched mouse immunoglobulin G (IgG) was used at the same concentration. For counting of neurons, five predetermined fields surrounding the center point of the coverslip were chosen by a blinded examiner and then digitally photographed under bright-field microscopy on an Olympus IX70 inverted scope with a Hamamatsu Color-chilled 3 charge-coupled device (CCD) camera (Hamamatsu Phototonics K.K. Japan). Both TUNEL-positive and TUNEL-negative neurons were counted by two blinded examiners utilizing Scion Image software (version 1.62c; Scion Corp., Frederick, Md.). For each treatment condition, five to seven independently treated coverslips from two or more independent experiments were counted, and the average percentage of TUNEL-positive neurons ± standard error was determined. More than 800 neurons were scored for TUNEL reactivity on each coverslip.
Blockade of apoptosis with NMDA glutamate receptor antagonists.
To assess the role of the glutamate receptors in mediating neuronal apoptosis, NT2.N cultures were pretreated with the noncompetitive NMDA glutamate receptor antagonist MK-801, the competitive antagonist D-AP5, the amino-3-hydroxy-5-methyl-4-isoazole propionate (AMPA) receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), or the nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester HCl (l-NAME) (reagents above from Tocris Cookson, Inc., Ballwin, Mo.) before application of the HIV-1-infected MDM supernatants.
To assess the potential role of neuronal CXCR4 or CCR5 chemokine receptors in neuronal apoptosis in the NT2.N system, we treated selected cultures with either an anti-CXCR4 monoclonal antibody (MAb) (12G5; courtesy of J. Hoxie) or an anti-CCR5 MAb (MAB182; R & D Systems, Inc.) at a concentration of 10 μg/ml before and during exposure to supernatants. Isotype-matched IgG2A (CXCR4) and IgG2B (CCR5) control antibodies were used at the same concentration.
Induction of apoptosis with HA14-1, a Bcl-2 inhibitor.
HA14-1 (Calbiochem) is a small (molecular weight = 409) nonpeptidic, dimethyl sulfoxide (DMSO)-soluble ligand of Bcl-2 that binds to a critical Bcl-2 surface pocket, effectively inhibiting the biological activity of Bcl-2 (64). This results in activation of the apoptosis cascade through activation of Apaf-1 and caspases (64). NT2.N cultures were preincubated in medium containing DMSO-solubilized HA14-1 (final DMSO concentration, 0.003%) or 0.003% DMSO alone (control) for 4 h prior to exposure to conditioned medium from MDM. After an additional 48 h of exposure to conditioned medium from HIV-infected and noninfected MDM and containing HA14-1, cultures were analyzed by TUNEL assay for detection of apoptotic neurons.
Statistical analysis.
The data were expressed as the mean ± standard error. Comparisons between experimental groups were performed with a Student's t test. Differences were considered statistically significant for P values < 0.05.
RESULTS
NT2.N neuron-astrocyte cultures as a model for HIV-1/MDM-induced neuronal apoptosis.
NT2.N neurons are morphologically and functionally similar to primary human CNS neurons and have been widely used as a model for studies of mechanisms of neuronal excitotoxicity and apoptosis (46, 52, 54, 56). To determine the ability of NT2.N neurons to model HIV-1-induced apoptosis, we first developed a panel of relevant primary isolates derived from the CSF of HIV-1-infected patients and then determined the ability of culture supernatants from infected macrophages to induce NT2.N apoptosis in mixed neuronal-astrocytic cultures. Several of these isolates were derived from patients who have undergone extensive neurological, neuropsychiatric, and neuroimaging studies to assess their clinical status (Table 1). Since chemokine receptor utilization preference has been suggested to predict potential for neuronal apoptosis (49, 71), we determined the chemokine receptor utilization for each isolate as well as its ability to replicate in macrophages. Two of the isolates, jago and doge, are from patients that met critieria for mild AIDS dementia complex (ADC stages 0.5 to 1.0) (10, 25).
TABLE 1.
Characteristics of the HIV-1 isolates used in this study
As expected, each of the primary CSF isolates exhibited macrophage-tropism. Isolates jago and doge utilize the CCR5 chemokine receptor for macrophage infection (R5 isolates), while tybe utilizes CXCR4 (X4 isolate) (69). The dualtropic R5/X4 macrophage-tropic prototype, 89.6, has been previously shown to induce neuronal apoptosis in primary human fetal brain cell cultures (49).
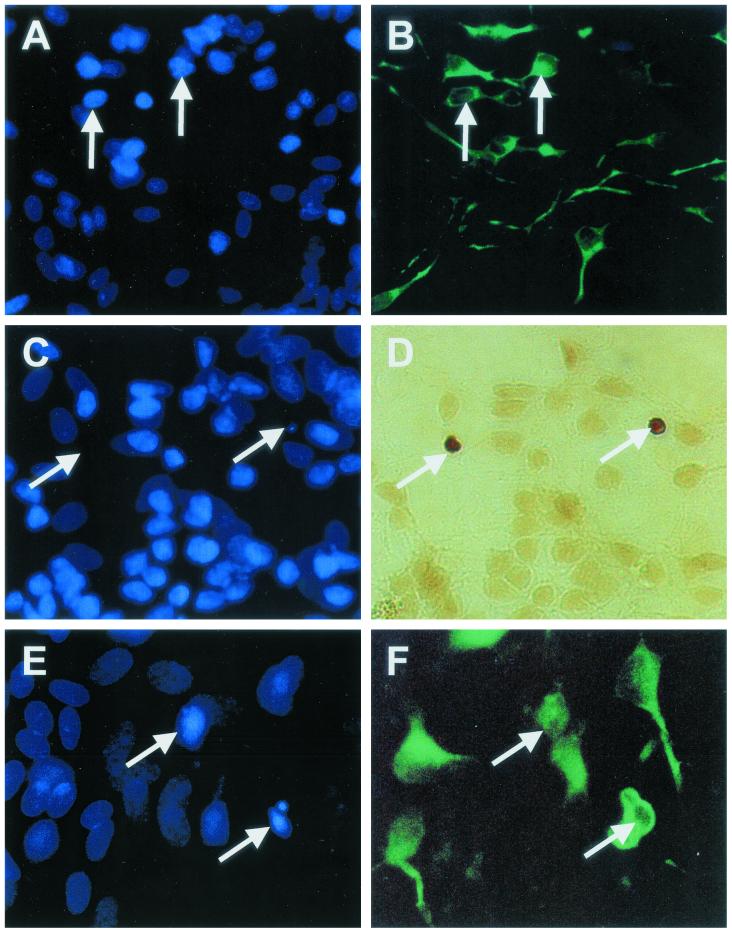
We infected MDM with a fixed amount (70 ng/106 cells) of cell-free virus inoculum, harvested culture supernatants at selected time points thereafter (Fig. 1), and applied the supernatants to NT2.N-astrocyte cultures. After 48 h, cultures were examined for apoptotic neurons by TUNEL assay and Hoechst staining (with MAP-2 labeling in some cases). As a positive control, we exposed the cultures to glutamate (1 mM) (19, 36, 56). We found that the TUNEL assay provided the most sensitive measure of neuronal apoptosis. Figure 2 shows identification of apoptotic nuclei in neurons in these mixed cultures. Figure 2A and B show nonapoptotic neurons colabeled with Hoechst nuclear stain (Fig. 2A) and for MAP-2 (Fig. 2B), a neuron-specific intermediate filament. Neuronal nuclei in these cultures were clearly identified by their intense Hoechst stain (compared with faintly stained astrocytes), small nuclei, and location in the focal plane above the astrocyte bed layer. Astrocytes were identified by GFAP labeling (not shown). Figure 2C and D show cultures colabeled with Hoechst and TUNEL. TUNEL labeling (Fig. 2D, arrows) blocked the nuclear labeling by Hoechst (Fig. 2C, arrows). However, MAP-2 labeling of neurons (Fig. 2F) colabeled with Hoechst (Fig. 2E) clearly identified neurons with the condensed (and often blebbed) nuclei seen in apoptosis. Therefore, we utilized TUNEL labeling to identify apoptotic neurons in the focal plane above the astrocyte bed.
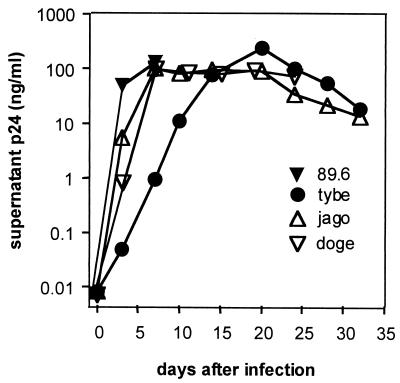
FIG. 1.
Replication of HIV-1 isolates derived from CSF and plasma in MDM. Monocytes isolated from PBMC were plated at 1.5 × 105 cells per cm2 and allowed to differentiate into macrophages. After 7 days, cultures were inoculated overnight with cell-free HIV-1 inoculum (70 ng of p24 antigen per 106 cells). Cultures were then washed, and the supernatant was collected periodically and assayed for p24 antigen. Collected supernatant was stored at −80°C and later applied to NT2.N-astrocyte cultures for analysis of neuronal apoptosis induction. The CSF isolates are tybe (X4), jago (R5), and doge (R5); 89.6 (R5/X4) is a PBMC isolate.
FIG. 2.
Identification of neuronal apoptosis in NT2.N-astrocyte cultures. NT2.N neurons were cultured on a feeder layer of rodent astrocytes for 5 weeks, exposed to HIV-1-infected or noninfected (control) MDM supernatants for 48 h, and then examined by TUNEL assay, Hoechst 33342 staining, or indirect immunofluorescence labeling with an anti-MAP-2 antibody, as described in Materials and Methods. Panels A and B show control cultures colabeled with Hoechst 33342 (A, nuclei) and MAP-2 (B, neurites). Panels C to F represent HIV/MDM-exposed cultures. Panel C shows loss of Hoechst nuclear staining in neurons colabeled by TUNEL (D, arrows). Panel E shows higher magnification of Hoechst-labeled nuclei with morphological features of apoptosis in neurons colabeled with MAP-2 (F). Magnification: panels A to D, ×600; panels E and F, ×1,000.
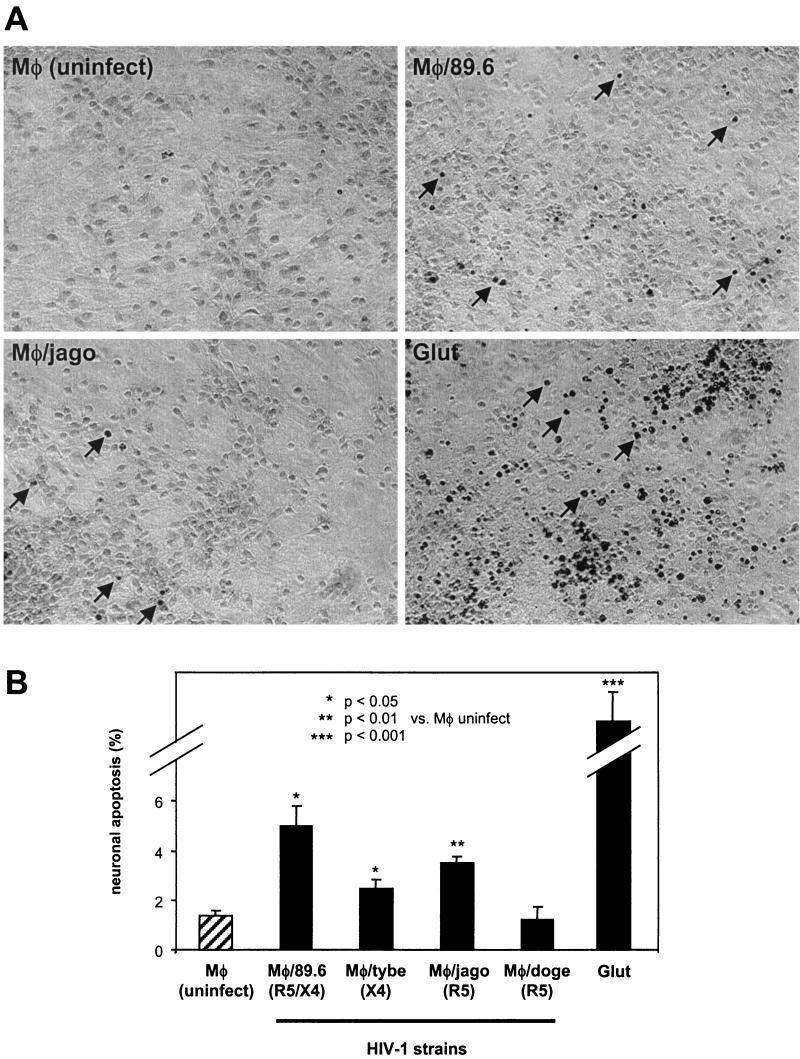
As shown in Fig. 3, significant levels of neuronal apoptosis were induced by MDM infected with each HIV-1 isolate except doge, as well as glutamate, in comparison with conditioned macrophage medium from mock-infected cells. Astrocyte apoptosis was rare in these cultures (<0.5%), as judged by GFAP identification of astrocytes (not shown). Interestingly, the level of neuronal apoptosis was not predicted by the level of replication of the isolate in macrophages, as judged by the p24 antigen level in the supernatants, which was similar for all isolates except tybe (Fig. 1). Isolate 89.6 consistently induced the highest levels of neuronal apoptosis, while doge (R5) failed to do so. Although some recent studies suggest that isolates that utilize CXCR4 (X4 and R5/X4 phenotypes) may have a greater ability to induce neuronal apoptosis in mixed glial-neuronal cultures (49, 71), our X4 isolate, tybe, although more virulent than doge (R5), was not consistently more virulent than jago (R5).
FIG.3.
Quantitation of neuronal apoptosis induced by HIV-1-infected MDM. NT2.N-astrocyte cultures were exposed to HIV-1-infected MDM supernatants or glutamate (Glut) (1 mM) and subjected to TUNEL assay as described in Materials and Methods. After 48 h, TUNEL-positive neurons were counted in five fields per coverslip by a blinded examiner. Each isolate was tested in two to three independent experiments, with two to three coverslips examined within each experiment. Results were expressed as the average percentage of TUNEL-positive neurons for all experiments ± standard error. (A) Phase-contrast photographs of TUNEL images from representative fields of neuronal-astrocyte cultures after exposure. Arrows indicate representative TUNEL-positive neurons. Magnification, ×200. (B) Average total percent neuronal apoptosis for all experiments. Total experiments examined were as follows: mock, n = 3; 89.6, n = 3; tybe, n = 3; jago, n = 3; doge, n = 2; glutamate, n = 2. Comparisons of means were made by Student's t test.
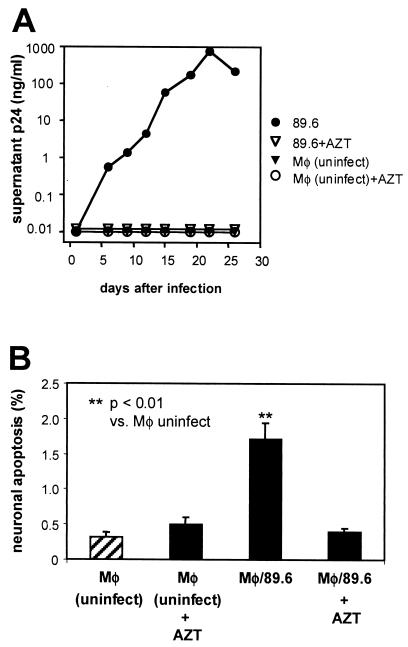
To determine whether apoptosis induction depended upon HIV replication in the MDM, we blocked virus replication with AZT (Fig. 4A) and compared the effects with those seen with productively infected MDM. As shown in Fig. 4B, both mock-infected and 89.6-inoculated, AZT-exposed MDM failed to induce neuronal apoptosis, while 89.6-infected MDM did. This demonstrates that our MDM required HIV replication and not merely exposure to the HIV inoculum for the production of soluble neurotoxic factors.
FIG. 4.
Prevention of neuronal apoptosis by inhibition of HIV-1 replication in MDM. MDM cultures were maintained with and without AZT (1 μM, added 1 h prior to HIV inoculation) and analyzed for p24 output (A). Supernatants (harvested at day 19) from each experimental condition were applied to NT2.N-astrocyte cultures, which were analyzed 48 h later for neuronal apoptosis (B), as in Fig. 3. Data represent the average percent neuronal apoptosis ± standard error from two independent experiments. Comparisons of means were made by Student's t test.
NMDA receptor blockade inhibits HIV-1-induced neuronal apoptosis.
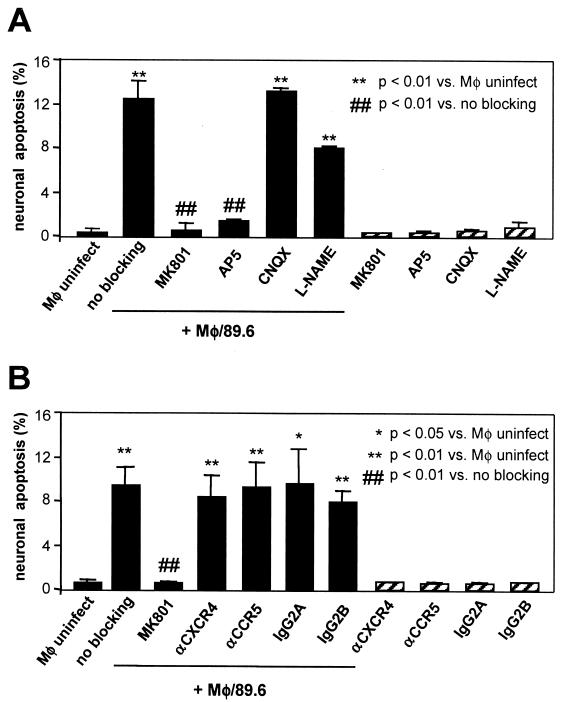
Similar to primary cortical neurons in culture, NT2.N neurons express functional NMDA-type glutamate receptors which mediate excitotoxic cell death in response to cognate ligands (23, 46, 56). Involvement of NMDA receptors in gp120-induced neuronal cell death is well established in studies showing inhibition of cell death by specific NMDA glutamate receptor antagonists and channel blockers (19, 33, 36). In addition, neurotoxicity of HIV-1-infected macrophage culture supernatants is also inhibited by NMDA channel blockade in primary neurons (19, 20). Therefore, to confirm that our model reflected similar pathways of apoptosis as identified in primary neurons, we tested selective NMDA and non-NMDA receptor antagonists. We found that the noncompetitive NMDA channel blocker MK801 and the competitive NMDA receptor antagonist D-AP5 inhibited HIV-1-induced apoptosis in NT2.N neurons (Fig. 5A). No protection was demonstrated by the non-NMDA glutamate receptor antagonist CNQX or the nitric oxide synthase inhibitor l-NAME.
FIG. 5.
Inhibition of HIV-1-induced neuronal apoptosis by NMDA receptor antagonists. Neuronal cultures were preincubated with various antagonists for 90 min prior to exposure to macrophage supernatants and examined by TUNEL assay 48 h later as described in Fig. 3. Solid bars represent cultures exposed to HIV/MDM medium (Mφ/89.6) or mock-infected (Mφ uninfect) medium. Hatched bars represent control cultures exposed to antagonist or antibody alone. (A) Individual cultures were preincubated with antagonists as follows: MK801 (10 μM), D-AP5 (1 mM), l-NAME (100 μM), or CNQX (30 μM). The data represent one of three independent replicate experiments. (B) Individual cultures were preincubated for 60 min with MK801 (10 μM), anti-CXCR4 MAb (12G5; 10 μg/ml), anti-CCR5 MAb (10 μg/ml), IgG2A isotype control antibody, or IgG2B isotype control antibody. The data represent average of two to three independent experiments. Results were expressed as mean ± standard error.
We next addressed the possibility that neuronal apoptosis might be the result of virion-mediated effects induced through association with neuronal chemokine receptors. We therefore tested anti-CXCR4 and anti-CCR5 antibodies for neuroprotection (21, 71). As shown in Fig. 5B, neither antibody afforded protection against 89.6, consistent with the inability of the envelope from strain 89.6 and naturally occurring HIV-1 strains in general to bind to chemokine receptors in the absence of CD4 (13, 69). Thus, NT2.N neurons respond similarly to primary fetal neurons to the neuroprotective effect of NMDA receptor blockade of toxicity from HIV-1-infected macrophages, and this toxicity is mediated primarily by neuronal NMDA receptors and not by neuronal chemokine receptors. These experiments are consistent with the previously demonstrated ability of HIV-1-infected MDM to release of neurotoxic levels of glutamate, which induce neuronal apoptosis through activation of NMDA receptors (18-20, 26).
Bcl-2 and Bcl-xL inhibit HIV-1/MDM-induced neuronal apoptosis.
Recent studies with various neuronal model systems indicate that induction of Bcl-2 family expression (Bax-α, Bcl-xL, and Bcl-2) in neurons regulates entry into the apoptosis cascade in response to a number of different inducers (6, 41). Since Bcl-2, Bcl-xL, and Bax-α are critical regulators of some, but not all, neuronal apoptosis pathways, we determined whether Bcl-2 and Bcl-xL might play a role in modulating HIV/MDM-induced neuronal apoptosis. Several studies indicate that certain neurons normally express each of these proteins, albeit to different levels (31, 32) and that Bcl-2 or Bcl-xL overexpression renders neurons resistant to apoptosis, while overexpression of Bax-α renders neurons susceptible (5, 9, 15, 55, 63). To determine the potential role of Bcl-2 and Bcl-xL in protecting neurons against HIV-1-induced apoptosis, we created several unique NT2.N cell lines by stable transfection with Bcl-2 and Bcl-xL expression plasmids.
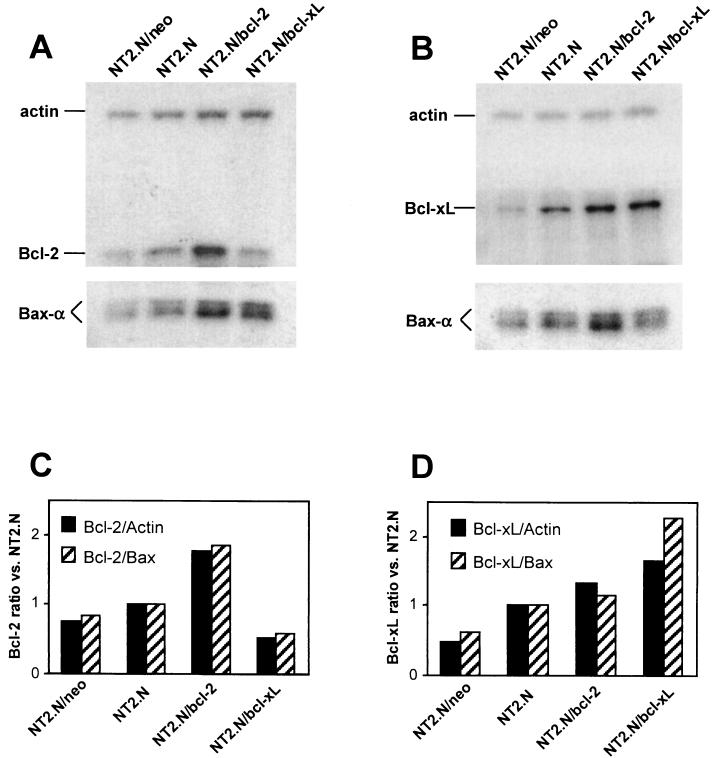
Figure 6 shows a Western blot analysis of Bcl-2 and Bcl-xL expression in the transfected NT2 cell lines in their fully differentiated (NT2.N) state. Endogenous basal Bcl-2 and Bcl-xL expression was seen in all NT2.N lines (Fig. 6A and B), but the level of expression of each protein was greater in lines stably transfected with the cognate plasmid in comparison with control NT2.N neurons. Quantitation of protein expression levels showed that the Bcl-2/actin and Bcl-xL/actin ratios, as well as the Bcl-2/Bax-α and Bcl-xL/Bax-α ratios, were greater in NT2.N/bcl-2 (Fig. 6C) and NT2.N/bcl-xL neurons (Fig. 6D), respectively. This relative increase in Bcl-2/Bax-α and Bcl-xL/Bax-α protein ratios, rather than absolute levels of either Bcl-2 or Bcl-xL, may be the determinant for entry into or diversion away from the apoptosis cascade (15, 36, 44, 50, 55) and would thus predict that both NT2.N/bcl-2 and NT2.N/bcl-xL neurons would be resistant to specific apoptosis signals.
FIG. 6.
Stable expression of Bcl-2 and Bcl-xL proteins in NT2.N cell lines. Cell lysates prepared from fully differentiated NT2.N neuronal cell lines were subjected to SDS-PAGE and Western blot analysis for Bcl-2, Bcl-xL, Bax, and actin expression. Stable cell lines were created by transfection with either Bcl-2 or Bcl-xL expression plasmids as described in Materials and Methods. NT2.N/neo cells were stably transfected with the plasmid pSV2neo. Equal amounts (25 μg) of protein were loaded into each lane in two separate gels and run in parallel. Membranes were cut to allow separate incubation with Bcl-2, Bcl-xL, Bax-α, and actin MAbs, and the resultant films were analyzed by densitometry to estimate relative protein amounts. (A) Bcl-2 expression. (B) Bcl-xL expression. (C) Bcl-2/Bax-α expression ratio. (D) Bcl-xL/Bax-α expression ratio. Representative results from one of two independent experiments are shown.
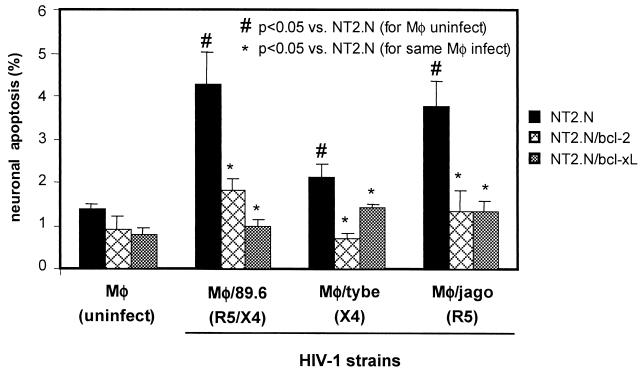
Compared with control NT2.N neurons, both NT2.N/bcl-2 and NT2.N/bcl-xL neurons were consistently resistant to HIV-1-infected macrophage supernatants, as shown in Fig. 7. In each experiment where significant levels of apoptosis were seen in the parental HIV-1/MDM-exposed NT2.N neurons, similarly exposed NT2.N/bcl-2 and NT2.N/bcl-xL neurons were significantly resistant (P < 0.01 to 0.05; Student's t test). Such neuroprotection by Bcl-2 and Bcl-xL is also seen in neurons exposed to glutamate (62), and partial protection was seen in our NT2.N/bcl-2 and NT2.N/bcl-xL neurons exposed to the high levels of glutamate (1 mM) used in control experiments (not shown). The neuroprotective effect in NT2.N/bcl-2 and NT2.N/bcl-xL neurons was seen for HIV-1 isolates of each chemokine receptor phenotype: X4/R5 (89.6), X4 (tybe), and R5 (jago).
FIG. 7.
Resistance of NT2.N/bcl-2 and NT2.N/bcl-xL neurons to HIV-1-induced apoptosis. NT2.N neurons derived from stably transfected NT2 cell lines were cultured on a feeder layer of astrocytes for 5 weeks, exposed to HIV-1/MDM culture supernatants, and then assayed by TUNEL for apoptotic neurons as described in the legend to Fig. 3. Results are shown as the relative percentage of neuronal apoptosis in NT2.N, NT2.N/bcl-2, and NT2.N/bcl-xL neurons similarly exposed to supernatants derived from MDM independently infected with three different HIV-1 isolates. Results are expressed as the mean percentage of TUNEL-positive neurons from two independent experiments ± standard error.
Bcl-2 inhibition sensitizes NT2.N/bcl-2 neurons to HIV/MDM-induced apoptosis.
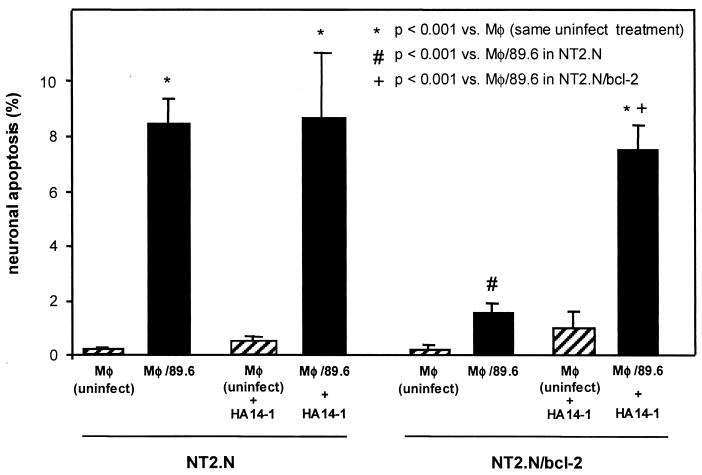
To confirm that neuroprotection in the NT2.N/bcl-2 neurons was due to biological activity of Bcl-2, we utilized a recently developed small molecule, the nonpeptidic Bcl-2 antagonist HA14-1, that binds directly to the Bcl-2 surface pocket to block Bcl-2 function (64). HA14-1 has been shown to induce apoptosis of HL-60 (human acute myleoid leukemia) cells overexpressing Bcl-2 protein, with a concomitant decrease in mitochondrial membrane potential and caspase activation, consistent with inhibition of the known biological activity of Bcl-2 (64). NT2.N cultures were preincubated with and maintained in HA14-1 during exposure to HIV/MDM supernatants. Based on published work (64) and on direct examination of cultures exposed to various concentrations of HA14-1, we selected a working concentration (30 μM) below concentrations observed to cause cytopathogenicity in control cultures (data not shown). As shown in Fig. 8, HA14-1 pretreatment of NT2.N/bcl-2 cultures resulted in levels of neuronal apoptosis comparable to that achieved in control NT2.N neurons exposed to HIV/MDM supernatants. This is consistent with protection against HIV-1/MDM-induced apoptosis specifically by Bcl-2 in these neurons. Interestingly, HA14-1 did not increase the level of HIV/macrophage-induced apoptosis in control NT2.N neurons, which express basal levels of endogenous Bcl-2. This suggests that increasing Bcl-2 expression in neurons over basal expression can afford protection against HIV/MDM-induced apoptosis.
FIG. 8.
Sensitization of NT2.N/bcl-2 neurons to HIV-infected MDM supernatants by the Bcl-2 antagonist, HA14-1. Select NT2.N/bcl-2 cultures were preincubated with HA14-1 (30 μM) for 4 h prior to exposure to HIV-infected MDM supernatants as described in the legend to Fig. 3 and maintained for another 48 h prior to TUNEL assay. Results are plotted as the mean percentage of apoptotic neurons from five coverslips from two independent experiments ± standard error.
DISCUSSION
In this study, we have developed a novel model of HIV/macrophage-induced neuronal apoptosis and demonstrated that Bcl-2 and Bcl-xL can inhibit neuronal apoptosis induced by HIV-1. This neuronal system recapitulates key features of HIV-induced excitotoxic (NMDA receptor/glutamate-mediated) neuronal apoptosis demonstrated in other human and nonhuman primary neuronal systems. In addition, we demonstrated that primary CNS isolates of either the R5 or X4 phenotype, as well as the prototypic primary R5/X4 peripheral blood isolate 89.6, induce neuronal apoptosis with various levels of efficiency that are not predictable by their chemokine receptor utilization. This is the first direct demonstration of the neuroprotective effects of Bcl-2 and Bcl-xL against HIV-1, and our study suggests that neurodegeneration in HIV-1 infection, like neurodegeneration in cerebral ischemia or stroke, may be targeted by strategies that modulate function of the Bcl-2 family. Our studies further suggest that such strategies may offer protection against HIV-1 isolates of varied chemokine receptor utilization phenotypes that may emerge throughout the course of infection in vivo.
Numerous studies have demonstrated that HIV-1-infected macrophages and microglia induce neuronal cell death in mixed primary cell culture systems through release of neurotoxins, and it is generally accepted that such a mechanism is largely responsible for neuronal death in HIV-1 infection in vivo (reviewed in references 17, 35, and 47). Our studies are consistent with previous reports demonstrating that the ability of HIV-1 isolates to induce neuronal apoptosis in vitro varies independently of the level of replication in macrophages. In contrast to others who have demonstrated that differences in the ability of HIV-1 strains to induce neuronal apoptosis may vary according to their chemokine receptor utilization preferences (49, 59, 71), we found no such association among a group of primary isolates that, although small, was derived from CNS tissue.
In both rodent and human culture systems, multiple investigators have shown neuroprotective effects of NMDA receptor blockade against toxicity induced by either HIV-1-infected macrophage supernatants or recombinant gp120, consistent with glutamate-mediated excitotoxicity (reviewed in references 27, 29, and 35). Our NT2.N culture system accurately reproduces responses seen in such primary neuronal-glial cell systems. NT2.N neurons express a variety of neuronal proteins, including MAP-1 and MAP-2, growth-associated protein (GAP43), synaptophysin, m1- and m2-type muscarinic acetylcholine receptors, amyloid precursor protein (APP), all three classes of neurofilaments, and NMDA and non-NMDA subtype glutamate receptors (37, 54, 65, 66, 70). This is a widely used model for human neuronal apoptosis and glutamate-mediated excitoxicity (23, 46, 56).
Our blocking experiments with glutamate receptor antagonists and chemokine receptor antibodies indicate that neurotoxicity in our system is likely mediated primarily by glutamate release from HIV-1-infected MDM, consistent with previous reports (26, 33, 42, 45). In addition, our experiments with AZT indicate that HIV-1 replication in MDM is necessary for induction of neuronal apoptosis in our model system. In total, these experiments thus indicate that neurotoxicity is dependent upon the release of soluble neurotoxic glutamate receptor agonists from MDM and that this release is induced by HIV-1 replication. Failure of either anti-CXCR4 or -CCR5 antibodies to prevent neuronal apoptosis suggests that there are not direct apoptosis-inducing effects in neurons of chemokine receptor ligands that may be released from HIV-1-infected MDM (HIV envelope or chemokines) in our culture system. Furthermore, our X4 isolate tybe, and our R5/X4 isolate 89.6 are strictly CD4 dependent, and direct toxicity from neuronal chemokine receptor binding by HIV-1 envelope from primary X4, R5, or R5/X4 isolates has not been demonstrated. Nonetheless, we cannot rule out the possibility HIV-1 envelope (either soluble envelope protein or virion-associated envelope) released from infected MDM may in turn bind to MDM to induce release of glutamate and other aforementioned neurotoxins that ultimately modulate glutamate release from glial cells (26; reviewed in reference 27).
Because neuronal-astrocytic interactions modulate neuronal cell function in the CNS, we performed our experiments with NT2.N-astrocyte cocultures, which thereby resemble primary mixed neuronal-glial culture systems. Neuronal cell death in such systems is generally much lower than in neuron-enriched cultures, due to a protective effect of astrocytes (48). Both in vitro and in vivo studies have demonstrated that HIV-associated death of significant numbers of neurons occurs by apoptosis, despite the possibility of multiple initiating cues, such as gp120, HIV-infected macrophage supernatants, Tat, Vpr, nitric oxide, and others (reviewed in references 29, 35, and 51). Several of these neurotoxins are known to mediate their effects ultimately through the activation of NMDA receptors. This suggests that rational neuroprotective strategies against HIV may be directed not only at the level of neurotoxin production (antiretrovirals and free radical scavengers) and at the level of the neuronal cell surface (NMDA receptors and chemokine receptors), but also at intracellular targets within the apoptosis cascade (Bcl-2 family proteins, caspases, cytochrome c, and others).
Although recent studies have demonstrated protective effects of Bcl-2 and Bcl-xL against neuronal apoptosis under a variety of conditions, including cerebral ischemia, growth factor deprivation, glucose deprivation, glutamate, and β-amyloid protein exposure (12, 32, 55, 60, 68), such a potential role for neuronal Bcl-2 or Bcl-xL in HIV-1-induced apoptosis has not been examined. Interestingly, Bcl-2 or Bcl-xL cannot protect neurons against all apoptosis inducers, including deprivation of some growth factors (2), suggesting the possibility of other neuronal death pathways not controlled by Bcl-2 and Bcl-xL (reviewed in reference 41). Our studies indicate a clear protective role for both Bcl-2 and Bcl-xL against HIV-1-induced neuronal apoptosis mediated by NMDA receptor activation. This is thought to be a major pathway for induction of neuronal apoptosis in vivo, although some studies suggest that other surface receptors may mediate toxic effects of HIV-1 proteins and even the α-chemokine SDF-1 (21, 28). We cannot rule out a minor role for activation of the extrinsic death receptor pathway in HIV/MDM-induced neuronal apoptosis, although our studies indicate a major role for the NMDA receptor-mediated, intrinsic Bcl-2 family-regulated pathway. Thus, although multiple mediators of neuronal apoptosis may be expressed by infected MDM, our studies indicate that Bcl-2 and Bcl-xL may block by a common mechanism triggered by multiple HIV/MDM-associated neurotoxins. Along these lines, it would be interesting to determine whether Bcl-2 or Bcl-xL also protects neurons against exposure to these agents.
Although our study indicates a protective role for Bcl-2 and Bcl-xL in neurons, it is unclear whether induction of neuronal apoptosis by HIV-1 involves induction of neuronal Bax-α, a proapoptotic member of the Bcl-2 family normally expressed at basal levels in neurons and other cells. Our demonstration of altered relative expression levels of antiapoptosis/proapoptosis bcl-2 gene products (Bcl-2/Bax-α and Bcl-xL/Bax-α ratios) in our stably transfected, HIV-resistant neurons are consistent with previous reports suggesting that these ratios, rather than absolute levels of Bcl-2, Bcl-xL, or Bax-α protein within the cell are determinants of neuroprotection (15, 38, 44, 50, 55). Bax-α is normally expressed in pyramidal neurons of the cortex and brain stem, hippocampal neurons, Purkinje cells of the cerebellum, sympathetic ganglia (31, 32), and during brain ischemia, some such subsets of neurons may also undergo apoptosis in association with increased Bax-α expression. Several of these brain regions also show significant levels of neuronal apoptosis in HIV-1 encephalitis (1, 16, 30, 59), but whether induction of neuronal Bax-α expression is involved is undetermined. One pathological study has suggested little difference in the levels of neuronal Bcl-2, Bcl-xL, and Bax-α (30) in HIV-1-infected brain, although this question clearly needs further examination. It is interesting to speculate that failure of certain neurons to increase their expression of endogenous Bcl-2 and/or Bcl-xL in response to HIV infection may put them at risk for apoptosis. Along these lines, exposure to a Bcl-2 antagonist did not increase the sensitivity of control neurons with basal Bcl-2 expression to HIV/macrophage-induced apoptosis, but did render Bcl-2-overexpressing neurons as sensitive as control neurons. Understanding the potential regulation of neuronal apoptosis in HIV-1-infected brain by Bcl-2 family proteins may offer clear targets for neuroprotective strategies for HIV-associated neurodegeneration.
Acknowledgments
We thank Lisa Wojcik and the University of Pennsylvania AIDS Clinical Trials Unit (ACTU) and the Center for AIDS Research (CFAR) for providing HIV isolates tybe, jago, and doge. We also thank Doris Shank (ACTU) and Joie Cutili for isolation of human primary peripheral monocytes and Arpita Agrawal for technical assistance. Finally, we thank Diane Merry, Francisco Gonzalez-Scarano, and Ron Collman for helpful discussions.
This work was supported by PHS grants NS35007, NS37651, and NS27405.
REFERENCES
- 1.Adle-Biassette, H., Y. Levy, M. Colombel, F. Poron, S. Natchev, C. Keohane, and F. Gray. 1995. Neuronal apoptosis in HIV infection in adults. Neuropathol. Appl. Neurobiol. 21:218-227. [DOI] [PubMed] [Google Scholar]
- 2.Allsopp, T. E., S. Wyatt, H. F. Paterson, and A. M. Davies. 1993. The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell 73:295-307. [DOI] [PubMed] [Google Scholar]
- 3.An, S. F., B. Giometto, T. Scaravilli, B. Tavolato, F. Gray, and F. Scaravilli. 1996. Programmed cell death in brains of HIV-1-positive AIDS and pre-AIDS patients. Acta Neuropathol. 91:169-173. [DOI] [PubMed] [Google Scholar]
- 4.Boersma, A. W. M., K. Nooter, H. Burger, C. J. Kortland, and G. Stoter. 1997. Bax upregulation is an early event in cisplatin-induced apoptosis in human testicular germ-cell tumor cell line NT2, as quantitated by flow cytometry. Cytometry 27:275-282. [DOI] [PubMed] [Google Scholar]
- 5.Boise, L. H., M. Gonzalez-Garcia, and C. E. Postema. 1993. Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74:597-608. [DOI] [PubMed] [Google Scholar]
- 6.Chao, D. T., and S. J. Korsmeyer. 1998. Bcl-2 family: regulators of cell death. Annu. Rev. Immunol. 16:395-419. [DOI] [PubMed] [Google Scholar]
- 7.Cheung, N. S., P. M. Beart, C. J. Pascoe, C. A. John, and O. Bernard. 2000. Human Bcl-2 protects against AMPA receptor-mediated apoptosis. J. Neurochem. 74:1613-1620. [DOI] [PubMed] [Google Scholar]
- 8.Collman, R., N. F. Hassan, R. Walker, B. Godfrey, J. Cutilli, J. C. Hastings, H. Friedman, S. D. Douglas, and N. Nathanson. 1989. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J. Exp. Med. 170:1149-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cregan, S. P., J. G. MacLaurin, C. G. Craig, G. S. Robertson, D. W. Nicholson, D. S. Park, and R. S. Slack. 1999. Bax-dependent caspase-3 activation is a key determinant in p53-induced apoptosis in neurons. J. Neurosci. 19:7860-7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders. 1996. Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder. Neurology 47:1247-1253. [DOI] [PubMed] [Google Scholar]
- 11.Dawson, V. L., T. Dawson, G. R. Uhl, and S. H. Snyder. 1993. Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical cultures. Proc. Natl. Acad. Sci. USA 90:3256-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deckwerth, T. L., J. L. Elliot, C. M. Knudson, E. M. Johnson, W. D. Snider, and S. J. Korsmeyer. 1996. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron 17:401-411. [DOI] [PubMed] [Google Scholar]
- 13.Edwards, T. G., T. L. Hoffman, F. Baribaud, S. Wyss, C. C. LaBranche, J. Romano, J. Adkinson, M. Sharron, J. A. Hoxie, and R. W. Doms. 2001. Relationships between CD4 independence, neutralization sensitivity, and exposure of a CD4-induced epitope in a human immunodeficiency virus type 1 envelope protein. J. Virol. 75:5230-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eldadah, B. A., and A. I. Faden. 2000. Caspase pathways, neuronal apoptosis, and CNS injury. J. Neurotrauma 17:811-829. [DOI] [PubMed] [Google Scholar]
- 15.Finucane, D. M., E. Bossy-Wetzel, N. J. Waterhouse, T. G. Cotter, and D. R. Green. 1999. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 274:2225-2233. [DOI] [PubMed] [Google Scholar]
- 16.Gelbard, H. A., H. J. James, L. R. Sharer, S. W. Perry, Y. Saito, A. M. Kazee, B. M. Blumberg, and L. G. Epstein. 1995. Apoptotic neurons in brains from paediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathol. Appl. Neurobiol. 21:208-217. [DOI] [PubMed] [Google Scholar]
- 17.Gendelman, H. E., S. A. Lipton, M. Tardieu, M. I. Bukrinsky, and H. S. L. M. Nottet. 1994. The neuropathogenesis of HIV-1 infection. J. Leukoc. Biol. 56:389-398. [DOI] [PubMed] [Google Scholar]
- 18.Giulian, D., K. Vaca, and C. A. Noonan. 1990. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science 250:1593-1596. [DOI] [PubMed] [Google Scholar]
- 19.Giulian, D., E. Wendt, K. Vaca, and C. A. Noonan. 1993. The envelope glycoprotein of human immunodeficiency virus type 1 stimulates release of neurotoxins from monocytes. Proc. Natl. Acad. Sci. USA 90:2769-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giulian, D., J. H. Yu, X. Li, D. Tom, J. Li, E. Wendt, S. N. Lin, R. Schwarcz, and C. Noonan. 1996. Study of receptor-mediated neurotoxins released by HIV-1-infected mononuclear phagocytes found in human brain. J. Neurosci. 16:3139-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hesselgesser, J., D. Taub, P. Baskar, M. Greenberg, J. Hoxie, D. L. Kolson, and R. Horuk. 1998. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1α is mediated by the chemokine receptor CXCR4. Curr. Biol. 8:595-598. [DOI] [PubMed] [Google Scholar]
- 22.Hockenberry, D., G. Nunez, C. Milliman, R. D. Schreiber, and S. J. Korsmeyer. 1990. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348:334-336. [DOI] [PubMed] [Google Scholar]
- 23.Itoh, T., A. Itoh, K. Horiuchi, and D. Pleasure. 1998. AMPA receptor-mediated excitotoxicity in human NT2-N neurons results from loss of intracellular Ca2+ homeostasis following marked elevation of intracellular Na+. J. Neurochem. 71:112-124. [DOI] [PubMed] [Google Scholar]
- 24.James, H. J., L. R. Sharer, Q. Zhang, H. G. Wang, L. G. Epstein, J. C. Reed, and H. A. Gelbard. 1999. Expression of caspase-3 in brains from paediatric patients with HIV-1 encephalitis. Neuropathol. Appl. Neurobiol. 25:380-386. [DOI] [PubMed] [Google Scholar]
- 25.Janssen, R. S., D. R. Cornblath, L. G. Epstein, R. P. Foa, J. C. McArthur, and R. W. Price. 1991. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Neurology 41:778-785. [DOI] [PubMed] [Google Scholar]
- 26.Jiang, Z.-G., C. Piggee, M. P. Heyes, C. Murphy, B. Quearry, M. Bauer, J. Zheng, H. E. Gendelman, and S. P. Markey. 2001. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. J. Neuroimmunol. 117:97-107. [DOI] [PubMed] [Google Scholar]
- 27.Kaul, M., G. A. Garden, and S. A. Lipton. 2001. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410:988-994. [DOI] [PubMed] [Google Scholar]
- 28.Kaul, M., and S. A. Lipton. 1999. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc. Natl. Acad. Sci. USA 96:8212-8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolson, D. L., E. Lavi, and F. Gonzalez-Scarano. 1998. The effects of human immunodeficiency virus in the central nervous system. Adv. Virus Res. 50:1-47. [DOI] [PubMed] [Google Scholar]
- 30.Krajewski, S., H. J. James, J. Ross, B. M. Blumberg, L. G. Epstein, H. E. Gendelman, S. Gummuluru, S. Dewhurst, L. R. Sharer, J. C. Reed, and H. A. Gelbard. 1997. Expression of pro- and anti-apoptosis gene products in brains from paediatric patients with HIV-1 encephalitis. Neuropathol. Appl. Neurobiol. 23:242-253. [PubMed] [Google Scholar]
- 31.Krajewski, S., M. Krajewska, A. Shabaik, T. Miyashita, H.-G. Wang, and J. C. Reed. 1994. Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am. J. Pathol. 145:1323-1336. [PMC free article] [PubMed] [Google Scholar]
- 32.Krajewski, S., J. K. Mai, M. Krajewski, M. Sikorska, M. J. Mossakowski, and J. C. Reed. 1995. Upregulation of bax protein levels in neurons following cerebral ischemia. J. Neurosci. 15:6364-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lannuzel, A., P.-M. Lledo, H. O. Lamghitnia, J.-D. Vincent, and M. Tardieu. 1995. HIV-1 envelope proteins gp120 and gp160 potentiate NMDA-induced [Ca2+]i increase, alter [Ca2+]i homeostasis and induce neurotoxicity in human embryonic neurons. Eur. J. Neurosci. 7:2285-2293. [DOI] [PubMed] [Google Scholar]
- 34.Li, H., H. Zhu, C.-J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 35.Lipton, S. A., and H. E. Gendelman. 1995. Dementia associated with the acquired immunodeficiency syndrome. N. Engl. J. Med. 332:934-940. [DOI] [PubMed] [Google Scholar]
- 36.Lipton, S. A., N. J. Sucher, P. K. Kaiser, and E. B. Dreyer. 1991. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron 7:111-118. [DOI] [PubMed] [Google Scholar]
- 37.Llanes, C., R. G. Collman, R. Hrin, and D. L. Kolson. 1995. Acetylcholinesterase expression in NTera 2 human neuronal cells: a model for developmental expression in the nervous system. J. Neurosci. Res. 42:791-802. [DOI] [PubMed] [Google Scholar]
- 38.Lohmann, C. M., A. A. League, W. S. Clark, D. Lawson, P. B. DeRose, and C. Cohen. 2000. Bcl-2:Bax and bcl-2:Bcl-x ratios by image cytometric quantitation of immunohistochemical expression in ovarian carcinoma: correlation with prognosis. Cytometry 42:61-66. [DOI] [PubMed] [Google Scholar]
- 39.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 40.McGinnis, K. M., M. E. Gnegy, and K. K. W. Wand. 1999. Endogenous Bax translocation in SH-SY5Y human neuroblastoma cells and cerebellar granule neurons undergoing apoptosis. J. Neurochem. 72:1899-1906. [DOI] [PubMed] [Google Scholar]
- 41.Merry, D. E., and S. J. Korsmeyer. 1997. Bcl-2 gene family in the nervous system. Annu. Rev. Neurosci. 20:245-267. [DOI] [PubMed] [Google Scholar]
- 42.Meucci, O., and R. J. Miller. 1996. gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-β1. J. Neurosci. 16:4080-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller, D. W. 2000. Immunobiology of the blood-brain barrier. J. Neurovirol. 5:570-578. [DOI] [PubMed] [Google Scholar]
- 44.Miyashita, T., S. Kitada, S. Krajewski, W. A. Horne, D. Delia, and J. C. Reed. 1995. Overexpression of the Bcl-2 protein increases the half-life of p21Bax. J. Biol. Chem. 270:26049-26052. [DOI] [PubMed] [Google Scholar]
- 45.Muller, W. E. G., H. C. Schroder, H. Ushijuma, J. Dapper, and J. Bormann. 1992. gp120 of HIV-1 induces apoptosis in rat cortical cell cultures: prevention by memantine. Eur. J. Pharmacol. 226:209-214. [DOI] [PubMed] [Google Scholar]
- 46.Munir, M., L. Lu, and P. McGonigle. 1995. Excitotoxic cell death and delayed rescue in human neurons derived from NT2 cells. J. Neurosci. 15:7847-7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Navia, B. A., and R. W. Price. 1998. Clinical and biologic features of the AIDS dementia complex, p. 229-240. In H. E. Gendelman, S. A. Lipton, L. Epstein, and S. Swindells (ed.), The neurology of AIDS. Chapman & Hall, New York, N.Y.
- 48.Nedergaard, M. 1994. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263:1768-1771. [DOI] [PubMed] [Google Scholar]
- 49.Ohagen, A., S. Ghosh, J. He, K. Huang, Y. Chen, M. Yuan, R. Osathanondh, S. Gartner, B. Shi, G. Shaw, and D. Gabuzda. 1999. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J. Virol. 73:897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oltavi, Z. N., C. L. Milman, and S. J. Korsmeyer. 1993. Bcl-2 heterodimerizes in vivo with a conserved homolog, bax, that accelerates programmed cell death. Cell 74:609-619. [DOI] [PubMed] [Google Scholar]
- 51.Patel, C. A., M. Mukhtar, and R. J. Pomerantz. 2000. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J. Virol. 74:9717-9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pekosz, A., J. Phillips, D. Pleasure, D. Merry, and F. Gonzalez-Scarano. 1996. Induction of apoptosis by La Crosse virus infection and role of neuronal differentiation and human bcl-2 expression in its prevention. J. Virol. 70:5329-5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petito, C. K., and B. Roberts. 1995. Evidence of apoptotic cell death in HIV encephalitis. Am. J. Pathol. 146:1121-1130. [PMC free article] [PubMed] [Google Scholar]
- 54.Pleasure, S. J., C. Page, and V. M-Y. Lee. 1992. Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J. Neurosci. 12:1802-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Putcha, G. V., M. Deshmukh, and E. M. Johnson. 1999. BAX translocation is a critical event in neuronal apoptosis: regulation by neuroprotectants, BCL-2 and caspases. J. Neurosci. 19:7476-7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rootwelt, T., M. Dunn, M. Yudkoff, T. Itoh, R. Almaas, and D. Pleasure. 1998. Hypoxic cell death in human NT2-N neurons: involvement of NMDA and non-NMDA glutamate receptors. J. Neurochem. 71:1544-1553. [DOI] [PubMed] [Google Scholar]
- 57.Saraste, A., and K. Pulkki. 2000. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 45:528-537. [DOI] [PubMed] [Google Scholar]
- 58.Selmaj, K. W., M. Farooq, W. T. Norton, C. S. Raine, and C. F. Brosnan. 1990. Proliferation of astrocytes in vitro in response to cytokines: a primary role for tumor necrosis factor. J. Immunol. 144:129-135. [PubMed] [Google Scholar]
- 59.Shi, B., U. de Girolami, J. He, S. Wang, A. Lorenzo, J. Busciglio, and D. Gabuzda. 1996. Apoptosis induced by HIV-1 infection of the central nervous system. J. Clin. Investig. 98:1979-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan, J., T. Town, A. Placzek, A. Kundtz, H. Yu, and M. Mullan. 1999. Bcl-xL inhibits apoptosis and necrosis produced by Alzheimer's β-amyloid1-40 peptide in PC12 cells. Neurosci. Lett. 272:5-8. [DOI] [PubMed] [Google Scholar]
- 61.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 62.Tyurin, V. A., Y. Y. Tyurina, P. J. Quinn, N. F. Schor, R. Balachandran, B. W. Day, and V. E. Kagan. 1998. Glutamate-induced cytotoxicity in PC12 pheochromocytoma cells: role of oxidation of phospholipids, glutathione and protein sulfhydryls revealed by Bcl-2 transfection. Mol. Brain Res. 60:270-281. [DOI] [PubMed] [Google Scholar]
- 63.Vekrellis, K., M. J. McCarthy, A. Watson, J. Whitfield, L. L. Rubin, and J. Ham. 1997. Bax promotes neuronal cell death and is downregulated during the development of the nervous system. Development 124:1239-1249. [DOI] [PubMed] [Google Scholar]
- 64.Wang, J.-L., D. Liu, Z.-J. Zhang, S. Shan, X. Han, S. M. Srinivasula, C. M. Croce, E. S. Alnemri, and Z. Huang. 2000. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl. Acad. Sci. USA 97:7124-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wertkin, A. M., R. S. Turner, S. J. Pleasure, T. E. Golde, S. G. Younkin, J. Q. Trojanowski, and V. M-Y. Lee. 1993. Human neurons derived from a teratocarcinoma cell line express solely the 695-amino acid amyloid precursor protein and produce intracellular β-amyloid or A4 peptides. Proc. Natl. Acad. Sci. USA 90:9513-9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolf, B. A., A. M. Wertkin, Y. C. Jolly, R. P. Yasuda, B. B. Wolfe, R. J. Konrad, D. Manning, S. Ravi, J. R. Williamson, and V. M.-Y. Lee. 1995. Muscarinic regulation of Alzheimer's disease amyloid precursor protein (APP) secretion and amyloid β-protein (Aβ) production in human neuronal NT2N cells. J. Biol. Chem. 270:4916-4922. [DOI] [PubMed] [Google Scholar]
- 67.Xiang, H., Y. Kinoshita, C. M. Knudson, S. J. Korsmeyer, P. A. Schwartzkroin, and R. S. Morrison. 1999. Bax involvement in p53-mediated neuronal cell death. J. Neurosci. 18:1363-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu, L. J., J. E. Lee, and R. G. Giffard. 1999. Overexpression of Bcl-2, Bcl-xL or Hsp70 in murine cortical astrocytes reduces injury of co-cultured neurons. Neurosci. Lett. 277:193-197. [DOI] [PubMed] [Google Scholar]
- 69.Yi, Y., S. Rana, J. D. Turner, N. Gaddis, and R. G. Collman. 1998. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J. Virol. 72:772-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Younkin, D. P., C. M. Tang, M. Hardy, U. R. Reddy, Q. Y. Shi, S. J. Pleasure, V. M. Lee, and D. Pleasure. 1993. Inducible expression of neuronal glutamate receptor channels in the NT2 human cell line. Proc. Natl. Acad. Sci. USA 90:2174-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng, J., A. Ghorpade, D. Niemann, R. L. Cotter, M. R. Thylin, L. Epstein, J. M. Swartz, R. B. Shepard, X. Liu, A. Nukuna, and H. E. Gendelman. 1999. Lymphotropic virions affect chemokine receptor-mediated neural signaling and apoptosis: implications for human immunodeficiency virus type 1-associated dementia. J. Virol. 73:8256-8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng, J., M. R. Thylin, A. Ghorpade, H. Xiong, Y. Persidsky, R. Cotter, D. Niemann, M. Che, Y.-C. Zeng, H. A. Gelbard, R. B. Shepard, J. M. Swartz, and H. E. Gendelman. 1999. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J. Neuroimmunol. 98:185-200. [DOI] [PubMed] [Google Scholar]