Abstract
Immunity to Mycobacterium tuberculosis requires a T helper 1 (Th1) cytokine balance accompanied by tumour necrosis factor-alpha (TNF-alpha), and activated macrophages. These facets of the immune response are sensitive to suppression by glucocorticoids (GC), which can reactivate and exacerbate tuberculosis in man and animals. Dehydroepiandrosterone (DHEA) and its derivative, 3beta,17beta androstenediol (AED), are reported to have antiglucocorticoid properties in vivo. We therefore investigated the effects of predetermined optimal doses of these compounds, on the course of pulmonary tuberculosis in an established model in BALB/c mice in which an early phase of Th1-mediated response accompanied by adrenal hyperplasia, is followed by a switch to Th2, progressive loss of TNF-alpha expression and disease progression. Both compounds were protective, particularly AED which caused a fall in bacterial counts and prolonged survival. These effects correlated with the appearance within 3 days of cellular infiltrates rich in cells expressing interleukin-2 (IL-2), IL-1alpha and TNF-alpha, and with partial suppression of the switch to IL-4 producing cells that occurred in controls. AED also caused enhanced development of granulomas at 14 days, and persistence of granuloma formation to 120 days, with a corresponding suppression of areas affected by pneumonia. Much of the therapeutic effect of AED and DHEA was obtained by treating for only the first 3 weeks, which is the phase of adrenal hyperplasia. These results suggest that the ratio of GC to anti-GC steroids may play a role in the pathogenesis of tuberculosis, and further investigation could lead to novel treatment strategies.
Full text
PDF

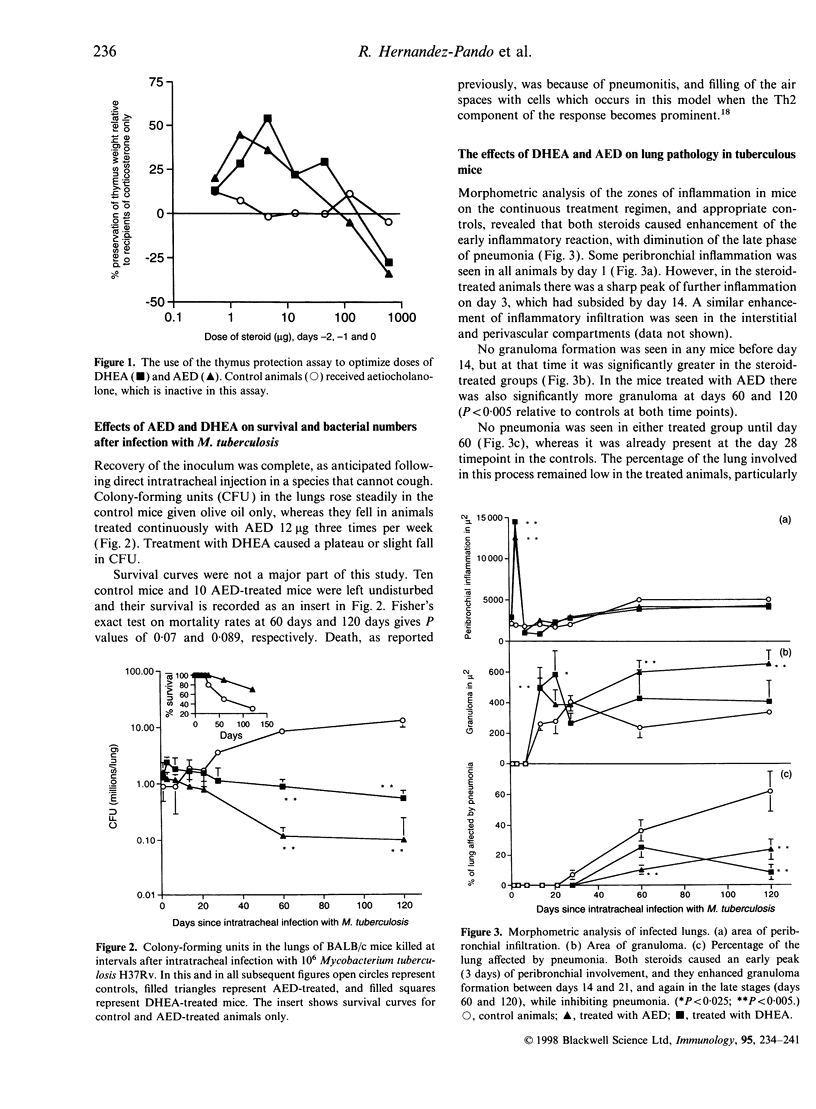
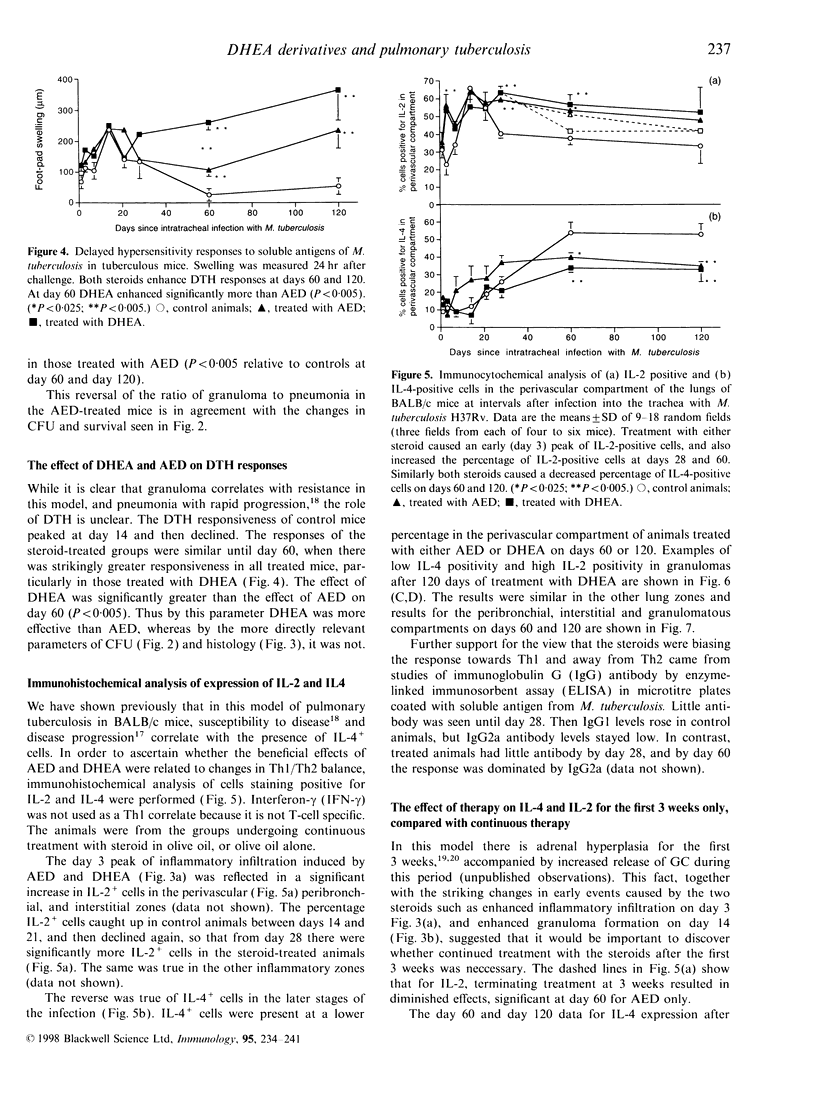

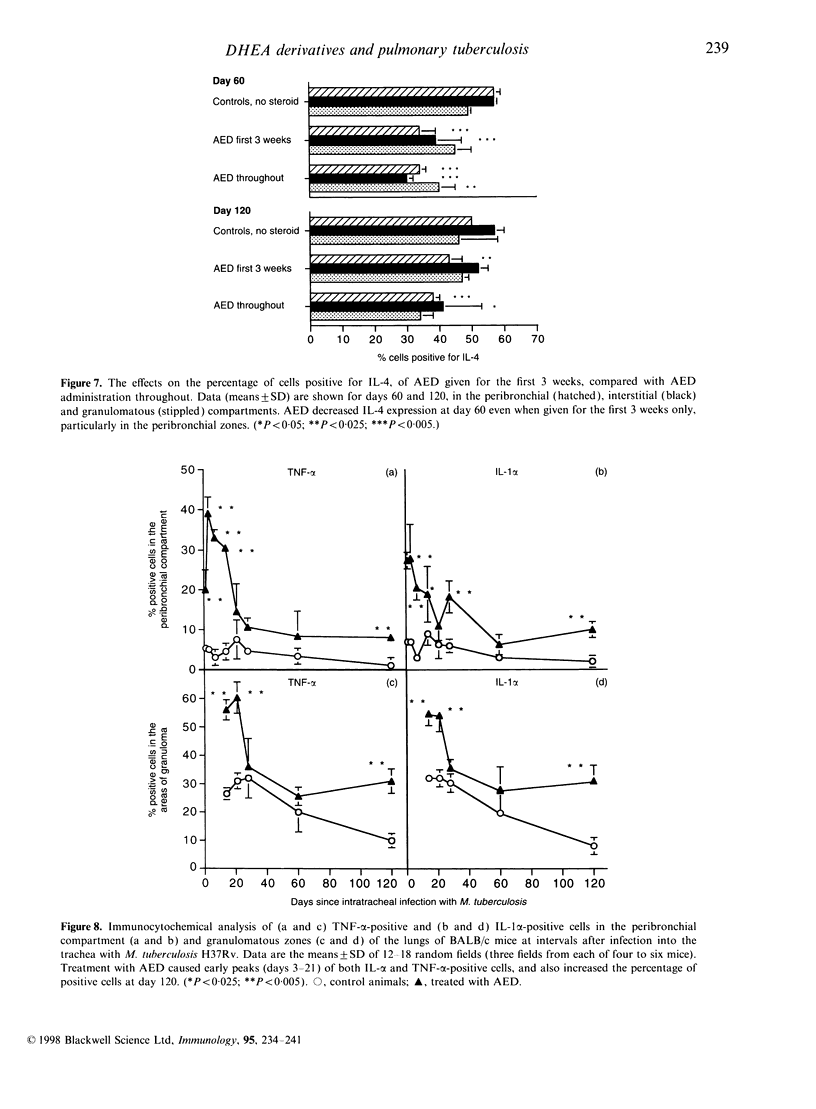


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blauer K. L., Poth M., Rogers W. M., Bernton E. W. Dehydroepiandrosterone antagonizes the suppressive effects of dexamethasone on lymphocyte proliferation. Endocrinology. 1991 Dec;129(6):3174–3179. doi: 10.1210/endo-129-6-3174. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Kristofic C. Regulation by corticosteroids of Th1 and Th2 cytokine production in human CD4+ effector T cells generated from CD45RO- and CD45RO+ subsets. J Immunol. 1995 Oct 1;155(7):3322–3328. [PubMed] [Google Scholar]
- Brown D. H., Sheridan J., Pearl D., Zwilling B. S. Regulation of mycobacterial growth by the hypothalamus-pituitary-adrenal axis: differential responses of Mycobacterium bovis BCG-resistant and -susceptible mice. Infect Immun. 1993 Nov;61(11):4793–4800. doi: 10.1128/iai.61.11.4793-4800.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. M., Magram J., Ferrante J., Orme I. M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J Exp Med. 1997 Jul 7;186(1):39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur J Immunol. 1989 Dec;19(12):2319–2325. doi: 10.1002/eji.1830191221. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A., Dowell T. A., Huang K., Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990 Apr 1;171(4):979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A., Ershler W. B., Maloney C., Li G. Z., Ryu S. Y. Altered regulation of IL-6 production with normal aging. Possible linkage to the age-associated decline in dehydroepiandrosterone and its sulfated derivative. J Immunol. 1993 Jun 15;150(12):5219–5230. [PubMed] [Google Scholar]
- Daynes R. A., Meikle A. W., Araneo B. A. Locally active steroid hormones may facilitate compartmentalization of immunity by regulating the types of lymphokines produced by helper T cells. Res Immunol. 1991 Jan;142(1):40–45. doi: 10.1016/0923-2494(91)90010-g. [DOI] [PubMed] [Google Scholar]
- Donald P. R., Beyers N. Adolescent tuberculosis. S Afr Med J. 1996 Mar;86(3):231–233. [PubMed] [Google Scholar]
- Fischer A., König W. Influence of cytokines and cellular interactions on the glucocorticoid-induced Ig (E, G, A, M) synthesis of peripheral blood mononuclear cells. Immunology. 1991 Oct;74(2):228–233. [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L., Chan J., Triebold K. J., Dalton D. K., Stewart T. A., Bloom B. R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993 Dec 1;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L., Goldstein M. M., Chan J., Triebold K. J., Pfeffer K., Lowenstein C. J., Schreiber R., Mak T. W., Bloom B. R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995 Jun;2(6):561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Garg M., Bondada S. Reversal of age-associated decline in immune response to Pnu-imune vaccine by supplementation with the steroid hormone dehydroepiandrosterone. Infect Immun. 1993 May;61(5):2238–2241. doi: 10.1128/iai.61.5.2238-2241.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guida L., O'Hehir R. E., Hawrylowicz C. M. Synergy between dexamethasone and interleukin-5 for the induction of major histocompatibility complex class II expression by human peripheral blood eosinophils. Blood. 1994 Oct 15;84(8):2733–2740. [PubMed] [Google Scholar]
- Hernandez-Pando R., Pavön L., Arriaga K., Orozco H., Madrid-Marina V., Rook G. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect Immun. 1997 Aug;65(8):3317–3327. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Pando R., Rook G. A. The role of TNF-alpha in T-cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology. 1994 Aug;82(4):591–595. [PMC free article] [PubMed] [Google Scholar]
- Kalimi M., Shafagoj Y., Loria R., Padgett D., Regelson W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Mol Cell Biochem. 1994 Feb 23;131(2):99–104. doi: 10.1007/BF00925945. [DOI] [PubMed] [Google Scholar]
- McCune R. M., Feldmann F. M., Lambert H. P., McDermott W. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med. 1966 Mar 1;123(3):445–468. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune R. M., Feldmann F. M., McDermott W. Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J Exp Med. 1966 Mar 1;123(3):469–486. doi: 10.1084/jem.123.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll I. Proliferative potential of different keratinocytes of plucked human hair follicles. J Invest Dermatol. 1995 Jul;105(1):14–21. doi: 10.1111/1523-1747.ep12312406. [DOI] [PubMed] [Google Scholar]
- Morales A. J., Nolan J. J., Nelson J. C., Yen S. S. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994 Jun;78(6):1360–1367. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]
- Morfin R., Courchay G. Pregnenolone and dehydroepiandrosterone as precursors of native 7-hydroxylated metabolites which increase the immune response in mice. J Steroid Biochem Mol Biol. 1994 Jul;50(1-2):91–100. doi: 10.1016/0960-0760(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Olsen N. J., Kovacs W. J. Gonadal steroids and immunity. Endocr Rev. 1996 Aug;17(4):369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- Padgett D. A., Loria R. M., Sheridan J. F. Endocrine regulation of the immune response to influenza virus infection with a metabolite of DHEA-androstenediol. J Neuroimmunol. 1997 Sep;78(1-2):203–211. doi: 10.1016/s0165-5728(97)00102-1. [DOI] [PubMed] [Google Scholar]
- Padgett D. A., Sheridan J. F., Loria R. M. Steroid hormone regulation of a polyclonal TH2 immune response. Ann N Y Acad Sci. 1995 Dec 29;774:323–325. doi: 10.1111/j.1749-6632.1995.tb17398.x-i1. [DOI] [PubMed] [Google Scholar]
- Ramírez F., Fowell D. J., Puklavec M., Simmonds S., Mason D. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J Immunol. 1996 Apr 1;156(7):2406–2412. [PubMed] [Google Scholar]
- Robel P., Baulieu E. E. Dehydroepiandrosterone (DHEA) is a neuroactive neurosteroid. Ann N Y Acad Sci. 1995 Dec 29;774:82–110. doi: 10.1111/j.1749-6632.1995.tb17374.x. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Hernandez-Pando R. The pathogenesis of tuberculosis. Annu Rev Microbiol. 1996;50:259–284. doi: 10.1146/annurev.micro.50.1.259. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Ainsworth M., Leveton C. A direct effect of glucocorticoid hormones on the ability of human and murine macrophages to control the growth of M. tuberculosis. Eur J Respir Dis. 1987 Oct;71(4):286–291. [PubMed] [Google Scholar]
- Suzuki T., Suzuki N., Daynes R. A., Engleman E. G. Dehydroepiandrosterone enhances IL2 production and cytotoxic effector function of human T cells. Clin Immunol Immunopathol. 1991 Nov;61(2 Pt 1):202–211. doi: 10.1016/s0090-1229(05)80024-8. [DOI] [PubMed] [Google Scholar]
- Wright B. E., Porter J. R., Browne E. S., Svec F. Antiglucocorticoid action of dehydroepiandrosterone in young obese Zucker rats. Int J Obes Relat Metab Disord. 1992 Aug;16(8):579–583. [PubMed] [Google Scholar]
- Wu C. Y., Sarfati M., Heusser C., Fournier S., Rubio-Trujillo M., Peleman R., Delespesse G. Glucocorticoids increase the synthesis of immunoglobulin E by interleukin 4-stimulated human lymphocytes. J Clin Invest. 1991 Mar;87(3):870–877. doi: 10.1172/JCI115092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Peretti E., Forest M. G. Pattern of plasma dehydroepiandrosterone sulfate levels in humans from birth to adulthood: evidence for testicular production. J Clin Endocrinol Metab. 1978 Sep;47(3):572–577. doi: 10.1210/jcem-47-3-572. [DOI] [PubMed] [Google Scholar]