Abstract
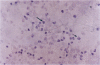
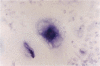
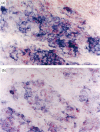
The microbicidal activity of macrophages in an inflammatory milieu has been related to the production of a large number of cytokins and intermediary metabolites of oxygen and nitrogen among them, nitric oxide (NO). Considering that granulomatous inflammation is predominantly composed of macrophages and epithelioid cells, we decided to investigate the participation of NO in this peculiar type of inflammation. Two models were used: glass cover slip implantation into the subcutaneous tissue of mice and, the inoculation of live bacillus Calmette-Guérin (BCG) into the footpad of the animals. Using a histochemical method for the detection of NO synthase and of the concentration of citrulin metabolized by cells obtained from cover slips implanted on different time intervals or BCG-activated peritoneal cells, it was possible to demonstrate that epithelioid cells do not produce NO. Cells from granuloma induced by BCG inoculation express NO synthase, with different degrees of reactivity with a higher intensity in the cytoplasm of cells located in the edge of the lesions. The expression of NO synthase in the cytoplasm of these cells decreases with the age of the lesions. It could also be demonstrated that in mice treated with l-name, an inhibitor of NO metabolism, the lesions induced by BCG lost the granulomatous architecture, were necrotic, and had a significant increase in the bacillary load of the lesion. These data allow us to conclude that NO production by macrophages is a determining factor in the organization of the granulomatous lesion and that it also controls the bacterial load in BCG-induced lesions in mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S. E., Källström L., Malm M., Miller-Larsson A., Axelsson B. Inhibition of nitric oxide synthase reduces Sephadex-induced oedema formation in the rat lung: dependence on intact adrenal function. Inflamm Res. 1995 Oct;44(10):418–422. doi: 10.1007/BF01757698. [DOI] [PubMed] [Google Scholar]
- Bray M., Prasad S., Dubay J. W., Hunter E., Jeang K. T., Rekosh D., Hammarskjöld M. L. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarero V. C., Junqueira V. B., Colepicolo P., Karnovsky M. L., Mariano M. Epithelial macrophages secrete a deactivating factor for superoxide release. J Cell Physiol. 1990 Dec;145(3):481–487. doi: 10.1002/jcp.1041450313. [DOI] [PubMed] [Google Scholar]
- Fujii E., Irie K., Ogawa A., Ohba K., Muraki T. Role of nitric oxide and prostaglandins in lipopolysaccharide-induced increase in vascular permeability in mouse skin. Eur J Pharmacol. 1996 Feb 22;297(3):257–263. doi: 10.1016/0014-2999(95)00758-x. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hiki K., Yui Y., Hattori R., Eizawa H., Kosuga K., Kawai C. Three regulation mechanisms of nitric oxide synthase. Eur J Pharmacol. 1991 Feb 25;206(2):163–164. doi: 10.1016/0922-4106(91)90026-e. [DOI] [PubMed] [Google Scholar]
- Ialenti A., Ianaro A., Moncada S., Di Rosa M. Modulation of acute inflammation by endogenous nitric oxide. Eur J Pharmacol. 1992 Feb 11;211(2):177–182. doi: 10.1016/0014-2999(92)90526-a. [DOI] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- Mariano M., Malucelli B. E. Defective phagocytic ability of epithelioid cells reversed by levamisole. J Pathol. 1980 Jan;130(1):33–36. doi: 10.1002/path.1711300105. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Ryan G. B., Spector W. G. Macrophage turnover in inflamed connective tissue. Proc R Soc Lond B Biol Sci. 1970 Aug 4;174(1040):269–292. doi: 10.1098/rspb.1970.0023. [DOI] [PubMed] [Google Scholar]
- Sacchi C. T., de Lemos A. P., Gorla M. C., Frasch C. E. Monoclonal antibody to serotype 17 of Neisseria meningitidis and their prevalence in Brazilian states. Rev Inst Med Trop Sao Paulo. 1995 Jan-Feb;37(1):1–5. doi: 10.1590/s0036-46651995000100001. [DOI] [PubMed] [Google Scholar]
- Saito S., Nakano M. Nitric oxide production by peritoneal macrophages of Mycobacterium bovis BCG-infected or non-infected mice: regulatory role of T lymphocytes and cytokines. J Leukoc Biol. 1996 Jun;59(6):908–915. doi: 10.1002/jlb.59.6.908. [DOI] [PubMed] [Google Scholar]
- Tomioka H., Sato K., Maw W. W., Saito H. The role of tumor necrosis factor, interferon-gamma, transforming growth factor-beta, and nitric oxide in the expression of immunosuppressive functions of splenic macrophages induced by Mycobacterium avium complex infection. J Leukoc Biol. 1995 Dec;58(6):704–712. doi: 10.1002/jlb.58.6.704. [DOI] [PubMed] [Google Scholar]
- Traub R. J., Solodkin A., Gebhart G. F. NADPH-diaphorase histochemistry provides evidence for a bilateral, somatotopically inappropriate response to unilateral hindpaw inflammation in the rat. Brain Res. 1994 May 30;647(1):113–123. doi: 10.1016/0006-8993(94)91405-2. [DOI] [PubMed] [Google Scholar]
- Woods M. L., 2nd, Mayer J., Evans T. G., Hibbs J. B., Jr Antiparasitic effects of nitric oxide in an in vitro murine model of Chlamydia trachomatis infection and an in vivo murine model of Leishmania major infection. Immunol Ser. 1994;60:179–195. [PubMed] [Google Scholar]