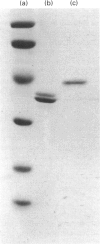
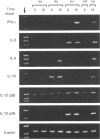
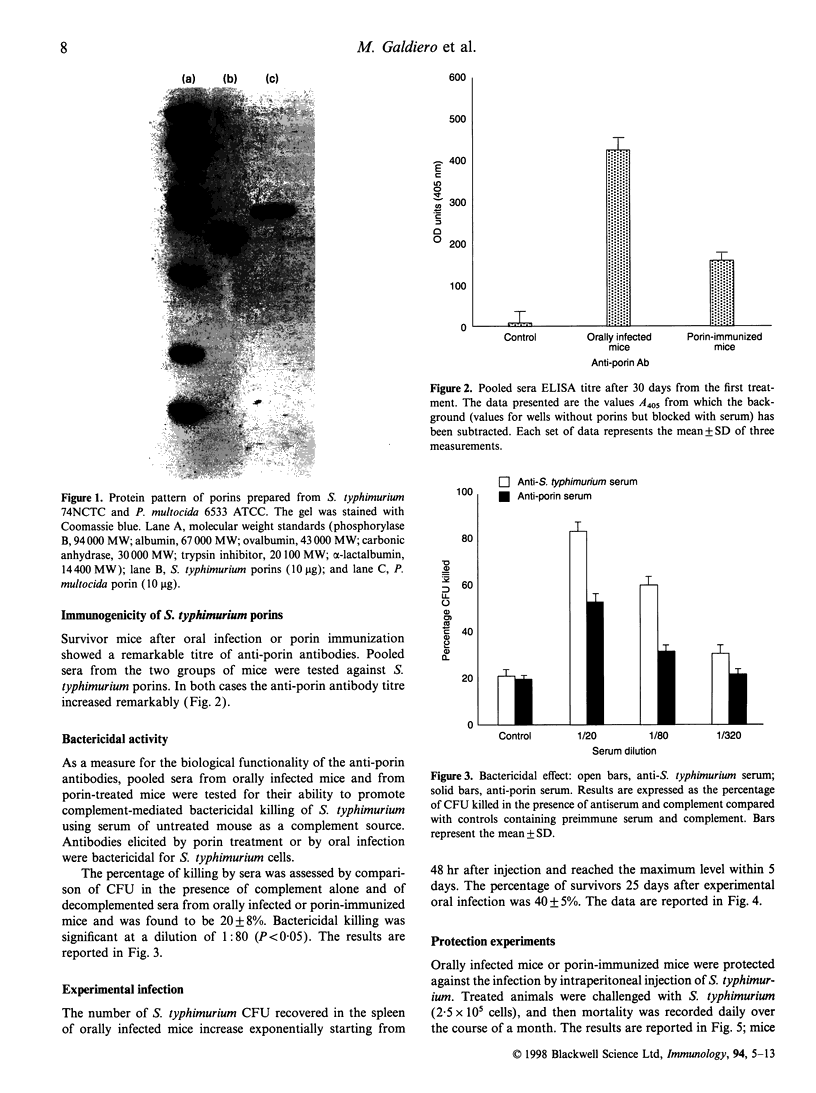
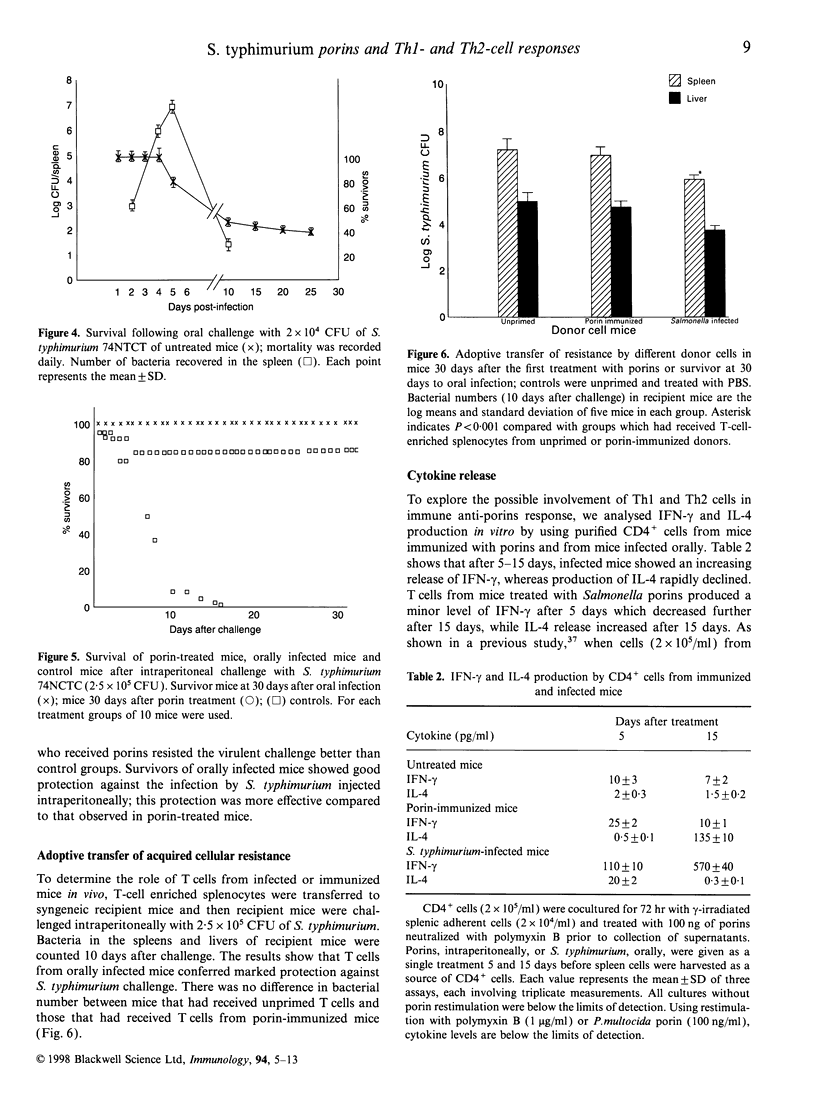
Abstract
In understanding the regulation of the specific immune response to Salmonella typhimurium, the role of a surface major component (porins) was studied. In this study we demonstrate that purified porins are able to induce a different response to that induced by the porins present on the S. typhimurium cell surface. Porin-treated or orally infected mice show anti-porin antibodies with bactericidal activity. The complete adoptive transfer of resistance to S. typhimurium is achieved only using splenic T cells from survivor mice after experimental infection. After stimulation with specific antigen in vitro CD4+ cells from porin-immunized mice released large amounts of interleukin-4 (IL-4), at a time when CD4+ cells from S. typhimurium-infected mice predominantly secreted interferon-gamma (IFN-gamma). Limiting dilution analysis showed that infection resulted in a higher precursor frequency of IFN-gamma-producing CD4+ T cells and a lower precursor frequency of IL-4-producing CD4+ T cells, while immunization with porins resulted in a higher precursor frequency of IL-4-producing cells and a low frequency of IFN-gamma-producing cells. Analysis of polymerase chain reaction-amplified cDNA from the spleens of infected mice revealed that IFN-gamma, IL-2 and IL-12 p40 mRNA were found 5 days after in vitro challenge and increased after 15 days; IL-10 expression was barely present after both 5 and 15 days, while IL-4 mRNA expression was not detected. In immunized mice, the IL-4 mRNA expression increased after 15 days, IFN-gamma mRNA expression disappeared entirely after 15 days, while IL-2, IL-10 and IL-12 mRNA remained relatively unchanged.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Van Der Meide P. H., Slayter M. V., Nacy C. A. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989 Jul 1;143(1):266–274. [PubMed] [Google Scholar]
- Berche P., Gaillard J. L., Sansonetti P. J. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987 Apr 1;138(7):2266–2271. [PubMed] [Google Scholar]
- Buchanan T. M., Eschenbach D. A., Knapp J. S., Holmes K. K. Gonococcal salpingitis is less likely to recur with Neisseria gonorrhoeae of the same principal outer membrane protein antigenic type. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):978–980. doi: 10.1016/0002-9378(80)91091-1. [DOI] [PubMed] [Google Scholar]
- Chevalier G., Duclohier H., Thomas D., Shechter E., Wróblewski H. Purification and characterization of protein H, the major porin of Pasteurella multocida. J Bacteriol. 1993 Jan;175(1):266–276. doi: 10.1128/jb.175.1.266-276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- D'Andrea A., Rengaraju M., Valiante N. M., Chehimi J., Kubin M., Aste M., Chan S. H., Kobayashi M., Young D., Nickbarg E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992 Nov 1;176(5):1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero F., de L'ero G. C., Benedetto N., Galdiero M., Tufano M. A. Release of cytokines induced by Salmonella typhimurium porins. Infect Immun. 1993 Jan;61(1):155–161. doi: 10.1128/iai.61.1.155-161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero M., Cipollaro de L'ero G., Donnarumma G., Marcatili A., Galdiero F. Interleukin-1 and interleukin-6 gene expression in human monocytes stimulated with Salmonella typhimurium porins. Immunology. 1995 Dec;86(4):612–619. [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Mutha S. S., Locksley R. M. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. S. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev. 1989 Dec;53(4):390–409. doi: 10.1128/mr.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Gros G., Ben-Sasson S. Z., Seder R., Finkelman F. D., Paul W. E. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990 Sep 1;172(3):921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni P., Skepper J. N., Hormaeche C. E. Effect of anti-tumor necrosis factor alpha antibodies on histopathology of primary Salmonella infections. Infect Immun. 1995 Sep;63(9):3674–3682. doi: 10.1128/iai.63.9.3674-3682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni P., Villarreal-Ramos B., Hormaeche C. E. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993 Sep;61(9):3981–3984. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni P., Villarreal-Ramos B., Hormaeche C. E. Role of T cells, TNF alpha and IFN gamma in recall of immunity to oral challenge with virulent salmonellae in mice vaccinated with live attenuated aro- Salmonella vaccines. Microb Pathog. 1992 Dec;13(6):477–491. doi: 10.1016/0882-4010(92)90014-f. [DOI] [PubMed] [Google Scholar]
- Matsui K., Arai T. The comparison of cell-mediated immunity induced by immunization with porin, viable cells and killed cells of Salmonella typhimurium. Microbiol Immunol. 1992;36(3):269–278. doi: 10.1111/j.1348-0421.1992.tb01664.x. [DOI] [PubMed] [Google Scholar]
- Mattern T., Thanhäuser A., Reiling N., Toellner K. M., Duchrow M., Kusumoto S., Rietschel E. T., Ernst M., Brade H., Flad H. D. Endotoxin and lipid A stimulate proliferation of human T cells in the presence of autologous monocytes. J Immunol. 1994 Oct 1;153(7):2996–3004. [PubMed] [Google Scholar]
- Miralles G. D., Stoeckle M. Y., McDermott D. F., Finkelman F. D., Murray H. W. Th1 and Th2 cell-associated cytokines in experimental visceral leishmaniasis. Infect Immun. 1994 Mar;62(3):1058–1063. doi: 10.1128/iai.62.3.1058-1063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Munkley A., Tinsley C. R., Virji M., Heckels J. E. Blocking of bactericidal killing of Neisseria meningitidis by antibodies directed against class 4 outer membrane protein. Microb Pathog. 1991 Dec;11(6):447–452. doi: 10.1016/0882-4010(91)90041-8. [DOI] [PubMed] [Google Scholar]
- Munn C. B., Ishiguro E. E., Kay W. W., Trust T. J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982 Jun;36(3):1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C., Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992 Feb;60(2):450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer F. A., Simonsen J. N., Chubb H., Slaney L., Kimata J., Bosire M., Ndinya-Achola J. O., Ngugi E. N. Epidemiologic evidence for the development of serovar-specific immunity after gonococcal infection. J Clin Invest. 1989 May;83(5):1472–1476. doi: 10.1172/JCI114040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner S. L., Wang Z. E., Hatam F., Scott P., Locksley R. M. TH1 and TH2 cell antigen receptors in experimental leishmaniasis. Science. 1993 Mar 5;259(5100):1457–1460. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- Rifkind D. Prevention by polymyxin B of endotoxin lethality in mice. J Bacteriol. 1967 Apr;93(4):1463–1464. doi: 10.1128/jb.93.4.1463-1464.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón M., Anguita J., Nakamura T., Fikrig E., Flavell R. A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997 Feb 3;185(3):461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxén H., Nurminen M., Kuusi N., Svenson S. B., Mäkelä P. H. Evidence for the importance of O antigen specific antibodies in mouse-protective Salmonella outer membrane protein (porin) antisera. Microb Pathog. 1986 Oct;1(5):433–441. doi: 10.1016/0882-4010(86)90005-7. [DOI] [PubMed] [Google Scholar]
- Srikumar R., Dahan D., Gras M. F., Saarinen L., Käyhty H., Sarvas M., Vogel L., Coulton J. W. Immunological properties of recombinant porin of Haemophilus influenzae type b expressed in Bacillus subtilis. Infect Immun. 1993 Aug;61(8):3334–3341. doi: 10.1128/iai.61.8.3334-3341.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Taylor P. W., Messner P., Parton R. Effect of the growth environment on cell-envelope components of Escherichia coli in relation to sensitivity to human serum. J Med Microbiol. 1981 Feb;14(1):9–19. doi: 10.1099/00222615-14-1-9. [DOI] [PubMed] [Google Scholar]
- Thatte J., Rath S., Bal V. Analysis of immunization route-related variation in the immune response to heat-killed Salmonella typhimurium in mice. Infect Immun. 1995 Jan;63(1):99–103. doi: 10.1128/iai.63.1.99-103.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatte J., Rath S., Bal V. Immunization with live versus killed Salmonella typhimurium leads to the generation of an IFN-gamma-dominant versus an IL-4-dominant immune response. Int Immunol. 1993 Nov;5(11):1431–1436. doi: 10.1093/intimm/5.11.1431. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Verheul A. F., Van Gaans J. A., Wiertz E. J., Snippe H., Verhoef J., Poolman J. T. Meningococcal lipopolysaccharide (LPS)-derived oligosaccharide-protein conjugates evoke outer membrane protein- but not LPS-specific bactericidal antibodies in mice: influence of adjuvants. Infect Immun. 1993 Jan;61(1):187–196. doi: 10.1128/iai.61.1.187-196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Zak K., Heckels J. E. Monoclonal antibodies to gonococcal outer membrane protein IB: use in investigation of the potential protective effect of antibodies directed against conserved and type-specific epitopes. J Gen Microbiol. 1986 Jun;132(6):1621–1629. doi: 10.1099/00221287-132-6-1621. [DOI] [PubMed] [Google Scholar]
- Wang Z. E., Reiner S. L., Zheng S., Dalton D. K., Locksley R. M. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med. 1994 Apr 1;179(4):1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzler L. M., Blake M. S., Barry K., Gotschlich E. C. Gonococcal porin vaccine evaluation: comparison of Por proteosomes, liposomes, and blebs isolated from rmp deletion mutants. J Infect Dis. 1992 Sep;166(3):551–555. doi: 10.1093/infdis/166.3.551. [DOI] [PubMed] [Google Scholar]
- Wetzler L. M., Blake M. S., Gotschlich E. C. Characterization and specificity of antibodies to protein I of Neisseria gonorrhoeae produced by injection with various protein I-adjuvant preparations. J Exp Med. 1988 Nov 1;168(5):1883–1897. doi: 10.1084/jem.168.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y., Kelso A., Cheers C. Differential activation of Brucella-reactive CD4+ T cells by Brucella infection or immunization with antigenic extracts. Infect Immun. 1995 Mar;63(3):969–975. doi: 10.1128/iai.63.3.969-975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]