Abstract
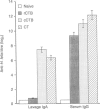
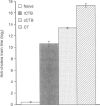
Cholera toxin is a potent oral mucosal adjuvant for enteric immunization. Several studies suggest that commercial cholera toxin B subunit (cCTB; purified from holotoxin) may be an effective non-toxic alternative for oral immunization. The present study was performed, using an infectious disease model, to determine if the oral mucosal adjuvanticity of CTB is dependent on contaminating holotoxin. Mice were orally immunized with Helicobacter felis sonicate and either cholera holotoxin, cCTB or recombinant cholera toxin B subunit (rCTB). Serum immunoglobulin G (IgG) and intestinal immunoglobulin A (IgA) antibody responses were determined and the mice were challenged with live H. felis to determine the degree of protective immunity induced. All orally immunized mice responded with serum IgG antibody titres regardless of the adjuvant used. However, only mice immunized with either holotoxin or the cCTB responded with an intestinal mucosal IgA response. Consistent with the production of mucosal antibodies, mice immunized with either holotoxin or cCTB as adjuvants were protected from challenge while mice receiving H. felis sonicate and rCTB all became infected. cCTB induced the accumulation of cAMP in mouse thymocytes at a level equal to 0.1% of that induced by holotoxin, whereas rCTB was devoid of any activity. These results indicate that CTB possesses no intrinsic mucosal adjuvant activity when administered orally. Therefore, when used as an oral adjuvant, CTB should also include small, non-toxic doses of cholera toxin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanchard T. G., Czinn S. J., Maurer R., Thomas W. D., Soman G., Nedrud J. G. Urease-specific monoclonal antibodies prevent Helicobacter felis infection in mice. Infect Immun. 1995 Apr;63(4):1394–1399. doi: 10.1128/iai.63.4.1394-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Lee A., Hazell S., Hu P., Li Y. Immunisation against gastric infection with Helicobacter species: first step in the prophylaxis of gastric cancer? Zentralbl Bakteriol. 1993 Sep;280(1-2):155–165. doi: 10.1016/s0934-8840(11)80952-7. [DOI] [PubMed] [Google Scholar]
- Czinn S. J., Cai A., Nedrud J. G. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine. 1993;11(6):637–642. doi: 10.1016/0264-410x(93)90309-l. [DOI] [PubMed] [Google Scholar]
- Czinn S. J., Nedrud J. G. Oral immunization against Helicobacter pylori. Infect Immun. 1991 Jul;59(7):2359–2363. doi: 10.1128/iai.59.7.2359-2363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tommaso A., Saletti G., Pizza M., Rappuoli R., Dougan G., Abrignani S., Douce G., De Magistris M. T. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996 Mar;64(3):974–979. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson B. L., Clements J. D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995 May;63(5):1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton K. A., Krakowka S. Chronic active gastritis due to Helicobacter pylori in immunized gnotobiotic piglets. Gastroenterology. 1992 Nov;103(5):1580–1586. doi: 10.1016/0016-5085(92)91181-3. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984 Jun;132(6):2736–2741. [PubMed] [Google Scholar]
- Farmer R. W., Harrington C. A., Brown D. H. A simple radioimmunoassay for 3',5', cyclic adenosine monophosphate. Anal Biochem. 1975 Apr;64(2):455–460. doi: 10.1016/0003-2697(75)90454-6. [DOI] [PubMed] [Google Scholar]
- Giannelli V., Fontana M. R., Giuliani M. M., Guangcai D., Rappuoli R., Pizza M. Protease susceptibility and toxicity of heat-labile enterotoxins with a mutation in the active site or in the protease-sensitive loop. Infect Immun. 1997 Jan;65(1):331–334. doi: 10.1128/iai.65.1.331-334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. C., Messer R. J., Cieplak W., Jr Role of trypsin-like cleavage at arginine 192 in the enzymatic and cytotonic activities of Escherichia coli heat-labile enterotoxin. Infect Immun. 1994 Oct;62(10):4270–4278. doi: 10.1128/iai.62.10.4270-4278.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashigucci K., Ogawa H., Ishidate T., Yamashita R., Kamiya H., Watanabe K., Hattori N., Sato T., Suzuki Y., Nagamine T. Antibody responses in volunteers induced by nasal influenza vaccine combined with Escherichia coli heat-labile enterotoxin B subunit containing a trace amount of the holotoxin. Vaccine. 1996 Feb;14(2):113–119. doi: 10.1016/0264-410x(95)00174-y. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lycke N., Czerkinsky C. Cholera toxin and cholera B subunit as oral-mucosal adjuvant and antigen vector systems. Vaccine. 1993 Sep;11(12):1179–1184. doi: 10.1016/0264-410x(93)90039-z. [DOI] [PubMed] [Google Scholar]
- Lebens M., Johansson S., Osek J., Lindblad M., Holmgren J. Large-scale production of Vibrio cholerae toxin B subunit for use in oral vaccines. Biotechnology (N Y) 1993 Dec;11(13):1574–1578. doi: 10.1038/nbt1293-1574. [DOI] [PubMed] [Google Scholar]
- Lee A., Chen M. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit-whole-cell vaccine. Infect Immun. 1994 Aug;62(8):3594–3597. doi: 10.1128/iai.62.8.3594-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K., Weltzin R., Thomas W. D., Jr, Kleanthous H., Ermak T. H., Soman G., Hill J. E., Ackerman S. K., Monath T. P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995 Jul;172(1):161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986 Oct;59(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- Lycke N., Karlsson U., Sjölander A., Magnusson K. E. The adjuvant action of cholera toxin is associated with an increased intestinal permeability for luminal antigens. Scand J Immunol. 1991 Jun;33(6):691–698. doi: 10.1111/j.1365-3083.1991.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Tsuji T., Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992 Sep;22(9):2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- Mbawuike I. N., Wyde P. R. Induction of CD8+ cytotoxic T cells by immunization with killed influenza virus and effect of cholera toxin B subunit. Vaccine. 1993 Sep;11(12):1205–1213. doi: 10.1016/0264-410x(93)90044-x. [DOI] [PubMed] [Google Scholar]
- McKenzie S. J., Halsey J. F. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J Immunol. 1984 Oct;133(4):1818–1824. [PubMed] [Google Scholar]
- Michetti P., Corthésy-Theulaz I., Davin C., Haas R., Vaney A. C., Heitz M., Bille J., Kraehenbuhl J. P., Saraga E., Blum A. L. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology. 1994 Oct;107(4):1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Nashar T. O., Amin T., Marcello A., Hirst T. R. Current progress in the development of the B subunits of cholera toxin and Escherichia coli heat-labile enterotoxin as carriers for the oral delivery of heterologous antigens and epitopes. Vaccine. 1993;11(2):235–240. doi: 10.1016/0264-410x(93)90023-q. [DOI] [PubMed] [Google Scholar]
- Sanchez J., Holmgren J. Recombinant system for overexpression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proc Natl Acad Sci U S A. 1989 Jan;86(2):481–485. doi: 10.1073/pnas.86.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S. Cautionary note on the use of the B subunit of cholera toxin as a ganglioside GM1 probe: detection of cholera toxin A subunit in B subunit preparations by a sensitive adenylate cyclase assay. J Cell Biochem. 1990 Mar;42(3):143–152. doi: 10.1002/jcb.240420305. [DOI] [PubMed] [Google Scholar]
- Szu S. C., Li X. R., Schneerson R., Vickers J. H., Bryla D., Robbins J. B. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect Immun. 1989 Dec;57(12):3823–3827. doi: 10.1128/iai.57.12.3823-3827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S., Funato H., Nagamine T., Aizawa C., Kurata T. Effectiveness of cholera toxin B subunit as an adjuvant for nasal influenza vaccination despite pre-existing immunity to CTB. Vaccine. 1989 Dec;7(6):503–505. doi: 10.1016/0264-410x(89)90273-9. [DOI] [PubMed] [Google Scholar]
- Tamura S., Ito Y., Asanuma H., Hirabayashi Y., Suzuki Y., Nagamine T., Aizawa C., Kurata T. Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J Immunol. 1992 Aug 1;149(3):981–988. [PubMed] [Google Scholar]
- Tamura S., Samegai Y., Kurata H., Nagamine T., Aizawa C., Kurata T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988 Oct;6(5):409–413. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- Tamura S., Yamanaka A., Shimohara M., Tomita T., Komase K., Tsuda Y., Suzuki Y., Nagamine T., Kawahara K., Danbara H. Synergistic action of cholera toxin B subunit (and Escherichia coli heat-labile toxin B subunit) and a trace amount of cholera whole toxin as an adjuvant for nasal influenza vaccine. Vaccine. 1994 Apr;12(5):419–426. doi: 10.1016/0264-410x(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Vajdy M., Lycke N. Y. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology. 1992 Mar;75(3):488–492. [PMC free article] [PubMed] [Google Scholar]
- Wilson A. D., Clarke C. J., Stokes C. R. Whole cholera toxin and B subunit act synergistically as an adjuvant for the mucosal immune response of mice to keyhole limpet haemocyanin. Scand J Immunol. 1990 Apr;31(4):443–451. doi: 10.1111/j.1365-3083.1990.tb02791.x. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Russell M. W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993 Jan;61(1):314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]