Abstract
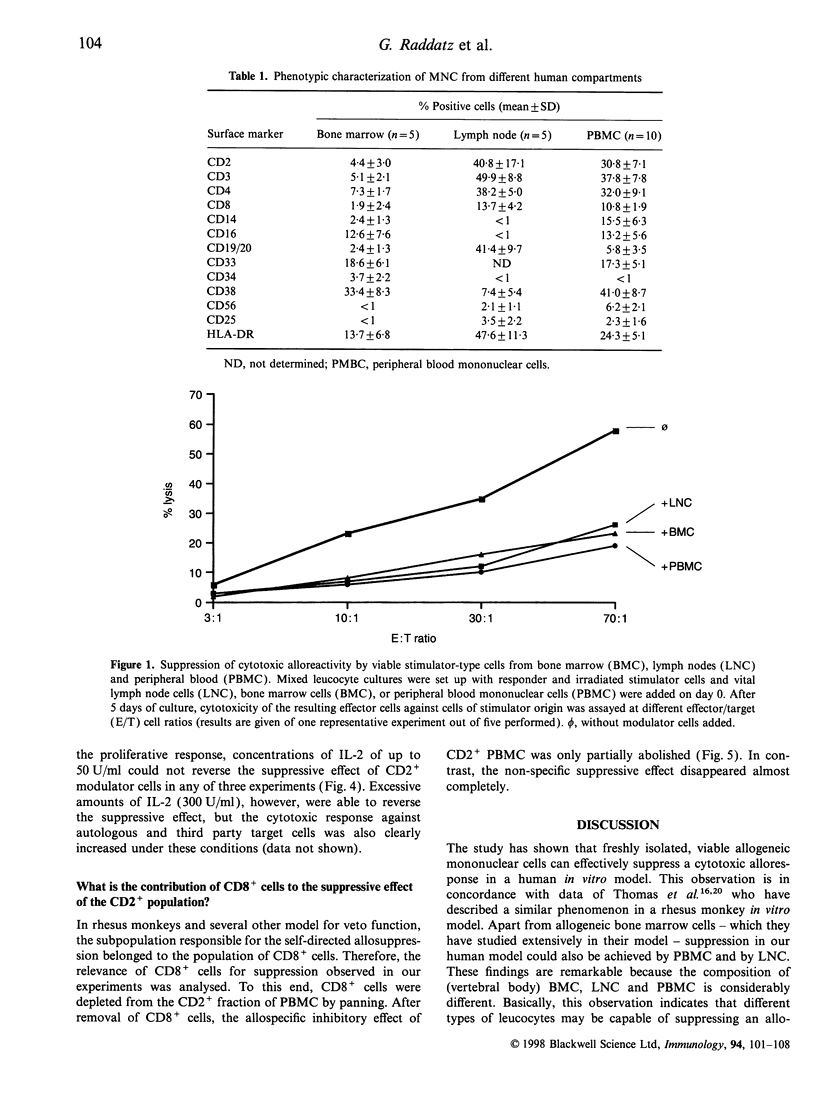
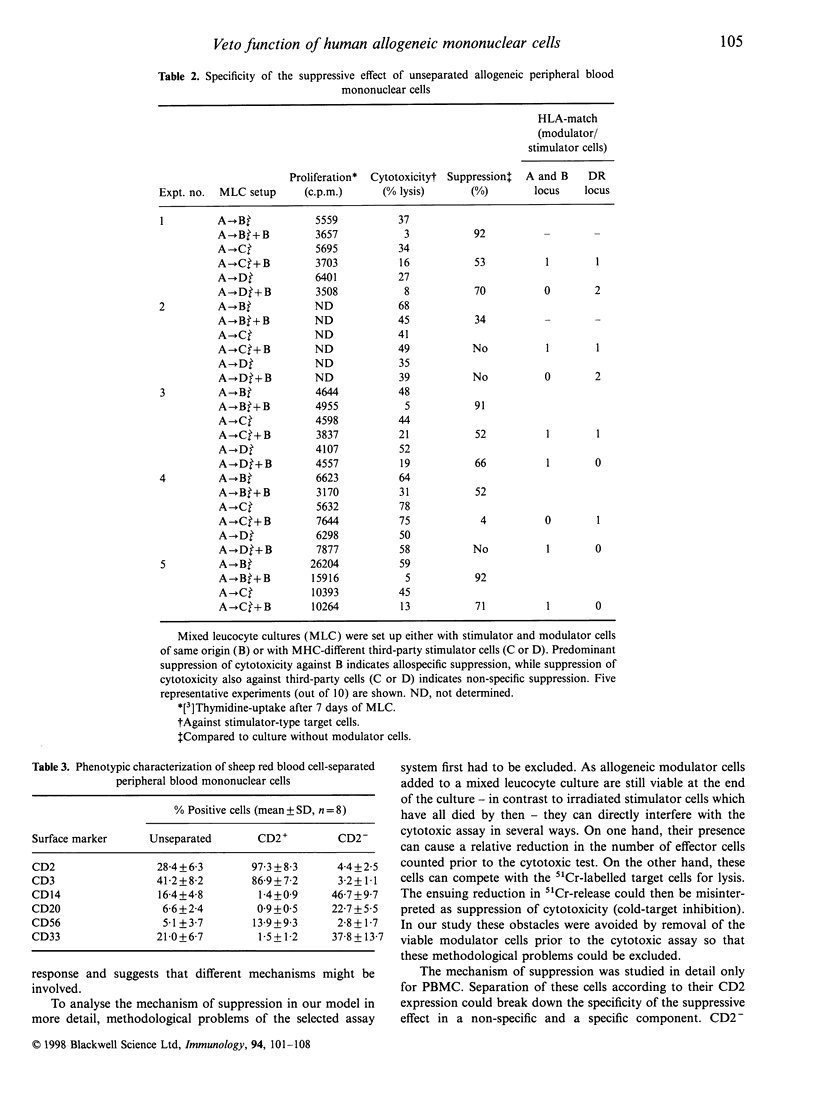
In animal models of organ transplantation, infusion of donor-derived leucocytes or bone marrow cells can support tolerance induction. To date, little is known about the suppressive effects of human allogeneic mononuclear cells on alloreactivity in the human system. To study this, mixed leucocyte cultures (MLC) were incubated in the presence and absence of viable allogeneic mononuclear cells (MNC) (modulator cells) of stimulator/donor origin, and the cytotoxic and proliferative potential of the resulting effector cells was determined. The experiments showed that: viable allogeneic MNC from bone marrow and from lymph nodes and peripheral blood (PBMC) were able to suppress allospecific cytotoxicity by an average of 60%; that allospecific as well as non-specific inhibitory effects could be observed with unseparated PBMC; that CD2+ PMNC showed predominantly allospecific inhibition of cytotoxicity with little effect on proliferation whereas CD2- PBMC showed non-specific inhibitory effects (both for cytotoxicity and proliferation), which could be eliminated by indomethacin; that addition of interleukin-2 (IL-2) up to 50 U/ml to the MLC could not reverse the inhibitory effect; and that selective removal of CD8+ cells from the CD2+ modulator population diminished the specific inhibitory effect only partially. These findings demonstrate that viable human MNC from different compartments can have a marked suppressive effect on alloreactivity in vitro. For peripheral blood mononuclear cells (PBMC) the data suggest that various mechanisms can contribute to allosuppression, including specific suppressive veto effects by CD2+ cells. Such inhibitory effects might be applicable in vivo for down-regulating allospecific cytotoxicity and to facilitate the acceptance of allografts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverly B., Kang S. M., Lenardo M. J., Schwartz R. H. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992 Jun;4(6):661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- Burlingham W. J., Grailer A. P., Fechner J. H., Jr, Kusaka S., Trucco M., Kocova M., Belzer F. O., Sollinger H. W. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation. 1995 Apr 27;59(8):1147–1155. [PubMed] [Google Scholar]
- Cassell D. J., Forman J. Regulation of the cytotoxic T lymphocyte response against Qa-1 alloantigens. J Immunol. 1990 Jun 1;144(11):4075–4081. [PubMed] [Google Scholar]
- Cassell D., Forman J. Two roles for CD4 cells in the control of the generation of cytotoxic T lymphocytes. J Immunol. 1991 Jan 1;146(1):3–10. [PubMed] [Google Scholar]
- Dallman M. J., Shiho O., Page T. H., Wood K. J., Morris P. J. Peripheral tolerance to alloantigen results from altered regulation of the interleukin 2 pathway. J Exp Med. 1991 Jan 1;173(1):79–87. doi: 10.1084/jem.173.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo F., Sasaki T., Sekiguchi Y., Haruyama T., Sato M., Abe K., Yoshinaga K. The role of monocytes and prostaglandin E in the regulation of mitogen response. Tohoku J Exp Med. 1981 Jan;133(1):119–120. doi: 10.1620/tjem.133.119. [DOI] [PubMed] [Google Scholar]
- Fast L. D. Generation and characterization of IL-2-activated veto cells. J Immunol. 1992 Sep 1;149(5):1510–1515. [PubMed] [Google Scholar]
- Fink P. J., Rammensee H. G., Bevan M. J. Cloned cytolytic T cells can suppress primary cytotoxic responses directed against them. J Immunol. 1984 Oct;133(4):1775–1781. [PubMed] [Google Scholar]
- Hambor J. E., Kaplan D. R., Tykocinski M. L. CD8 functions as an inhibitory ligand in mediating the immunoregulatory activity of CD8+ cells. J Immunol. 1990 Sep 15;145(6):1646–1652. [PubMed] [Google Scholar]
- Heeg K., Wagner H. Induction of peripheral tolerance to class I major histocompatibility complex (MHC) alloantigens in adult mice: transfused class I MHC-incompatible splenocytes veto clonal responses of antigen-reactive Lyt-2+ T cells. J Exp Med. 1990 Sep 1;172(3):719–728. doi: 10.1084/jem.172.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holda J. H., Maier T., Claman H. N. Murine graft-versus-host disease across minor barriers: immunosuppressive aspects of natural suppressor cells. Immunol Rev. 1985 Dec;88:87–105. doi: 10.1111/j.1600-065x.1985.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Hambor J. E., Tykocinski M. L. An immunoregulatory function for the CD8 molecule. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8512–8515. doi: 10.1073/pnas.86.21.8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman C. L., Colson Y. L., Wren S. M., Watkins S., Simmons R. L., Ildstad S. T. Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood. 1994 Oct 15;84(8):2436–2446. [PubMed] [Google Scholar]
- Koide J., Engleman E. G. Differences in surface phenotype and mechanism of action between alloantigen-specific CD8+ cytotoxic and suppressor T cell clones. J Immunol. 1990 Jan 1;144(1):32–40. [PubMed] [Google Scholar]
- Lagoo-Deenadayalan S., Lagoo A. S., Lemons J. A., Lorenz H. M., Bass J. D., McDaniel D. O., Hardy K. J., Barber W. H. Donor specific bone marrow cells suppress lymphocyte reactivity to donor antigens and differentially modulate TH1 and TH2 cytokine gene expression in the responder cell population. Transpl Immunol. 1995 Jun;3(2):124–134. doi: 10.1016/0966-3274(95)80039-5. [DOI] [PubMed] [Google Scholar]
- Le Gros G., Ben-Sasson S. Z., Seder R., Finkelman F. D., Paul W. E. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990 Sep 1;172(3):921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. R., Sheng-Tanner X., Miller R. G. Rapid and long-term changes to host cytotoxic T lymphocyte precursors reactive to donor antigens caused by intravenous injection of histoincompatible lymphocytes. Transplantation. 1992 Jul;54(1):125–129. doi: 10.1097/00007890-199207000-00022. [DOI] [PubMed] [Google Scholar]
- Martin P. J. Prevention of allogeneic marrow graft rejection by donor T cells that do not recognize recipient alloantigens: potential role of a veto mechanism. Blood. 1996 Aug 1;88(3):962–969. [PubMed] [Google Scholar]
- Mathew J. M., Carreno M., Fuller L., Ricordi C., Tzakis A., Esquenazi V., Miller J. Modulatory effects of human donor bone marrow cells on allogeneic cellular immune responses. Transplantation. 1997 Mar 15;63(5):686–692. doi: 10.1097/00007890-199703150-00013. [DOI] [PubMed] [Google Scholar]
- Miller R. G. An immunological suppressor cell inactivating cytotoxic T-lymphocyte precursor cells recognizing it. Nature. 1980 Oct 9;287(5782):544–546. doi: 10.1038/287544a0. [DOI] [PubMed] [Google Scholar]
- Qi Y., Berg R., Singleton M. A., Debrick J. E., Staerz U. D. Hybrid antibody mediated veto of cytotoxic T lymphocyte responses. J Exp Med. 1996 May 1;183(5):1973–1980. doi: 10.1084/jem.183.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammensee H. G., Kroschewski R., Frangoulis B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989 Jun 15;339(6225):541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- Rammensee H. G. Maintenance of self tolerance in CD4+ T lymphocytes by antigen presentation on resting B cells--a hypothesis. Bone Marrow Transplant. 1991;7 (Suppl 1):26–28. [PubMed] [Google Scholar]
- Roser B. J. Cellular mechanisms in neonatal and adult tolerance. Immunol Rev. 1989 Feb;107:179–202. doi: 10.1111/j.1600-065x.1989.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Saffran D. C., Singhal S. K. Suppression of mixed lymphocyte reactivity by murine bone-marrow-derived suppressor factor--inhibition of proliferation due to a deficit in IL-2 production. Transplantation. 1991 Oct;52(4):685–690. [PubMed] [Google Scholar]
- Sambhara S. R., Miller R. G. Programmed cell death of T cells signaled by the T cell receptor and the alpha 3 domain of class I MHC. Science. 1991 Jun 7;252(5011):1424–1427. doi: 10.1126/science.1828618. [DOI] [PubMed] [Google Scholar]
- Schlitt H. J., Kanehiro H., Raddatz G., Steinhoff G., Richter N., Nashan B., Ringe B., Wonigeit K., Pichlmayr R. Persistence of donor lymphocytes in liver allograft recipients. Transplantation. 1993 Oct;56(4):1001–1007. doi: 10.1097/00007890-199310000-00042. [DOI] [PubMed] [Google Scholar]
- Schlitt H. J., Raddatz G., Steinhoff G., Wonigeit K., Pichlmayr R. Passenger lymphocytes in human liver allografts and their potential role after transplantation. Transplantation. 1993 Oct;56(4):951–955. doi: 10.1097/00007890-199310000-00033. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Stanojević-Bakić N., Vucković-Dekić L., Susnjar S., Spuzić I. In vitro effect of indomethacin on mitogen-induced lymphoproliferative response in lung cancer patients. Neoplasma. 1992;39(2):129–132. [PubMed] [Google Scholar]
- Sun J., McCaughan G. W., Gallagher N. D., Sheil A. G., Bishop G. A. Deletion of spontaneous rat liver allograft acceptance by donor irradiation. Transplantation. 1995 Aug 15;60(3):233–236. doi: 10.1097/00007890-199508000-00004. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Maki T. Prolongation of mouse skin allograft survival by cloned veto suppressor cells. Transplant Proc. 1991 Feb;23(1 Pt 1):192–193. [PubMed] [Google Scholar]
- Thomas J. M., Carver F. M., Cunningham P. R., Olson L. C., Thomas F. T. Kidney allograft tolerance in primates without chronic immunosuppression--the role of veto cells. Transplantation. 1991 Jan;51(1):198–207. doi: 10.1097/00007890-199101000-00032. [DOI] [PubMed] [Google Scholar]
- Thomas J. M., Carver F. M., Kasten-Jolly J., Haisch C. E., Rebellato L. M., Gross U., Vore S. J., Thomas F. T. Further studies of veto activity in rhesus monkey bone marrow in relation to allograft tolerance and chimerism. Transplantation. 1994 Jan;57(1):101–115. doi: 10.1097/00007890-199401000-00018. [DOI] [PubMed] [Google Scholar]
- Thomas J. M., Verbanac K. M., Carver F. M., Kasten-Jolly J., Haisch C. E., Gross U., Smith J. P. Veto cells in transplantation tolerance. Clin Transplant. 1994 Apr;8(2 Pt 2):195–203. [PubMed] [Google Scholar]
- Thomas J. M., Verbanac K. M., Smith J. P., Kasten-Jolly J., Gross U., Rebellato L. M., Haisch C. E., Carver F. M., Thomas F. T. The facilitating effect of one-DR antigen sharing in renal allograft tolerance induced by donor bone marrow in rhesus monkeys. Transplantation. 1995 Jan 27;59(2):245–255. [PubMed] [Google Scholar]
- Thomson A. W., Lu L., Murase N., Demetris A. J., Rao A. S., Starzl T. E. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells. 1995 Nov;13(6):622–639. doi: 10.1002/stem.5530130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uberti J., Martilotti F., Chou T. H., Kaplan J. Human lymphokine activated killer (LAK) cells suppress generation of allospecific cytotoxic T cells: implications for use of LAK cells to prevent graft-versus-host disease in allogeneic bone marrow transplantation. Blood. 1992 Jan 1;79(1):261–268. [PubMed] [Google Scholar]
- Van Vlasselaer P., Niki T., Strober S. Identification of a factor(s) from cloned murine natural suppressor cells that inhibits IL-2 secretion during antigen-driven T cell activation. Cell Immunol. 1991 Dec;138(2):326–340. doi: 10.1016/0008-8749(91)90157-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Hirayama M., Genyea C., Kaplan J. TGF-beta mediates natural suppressor activity of IL-2-activated lymphocytes. J Immunol. 1994 Apr 15;152(8):3842–3847. [PubMed] [Google Scholar]