Abstract
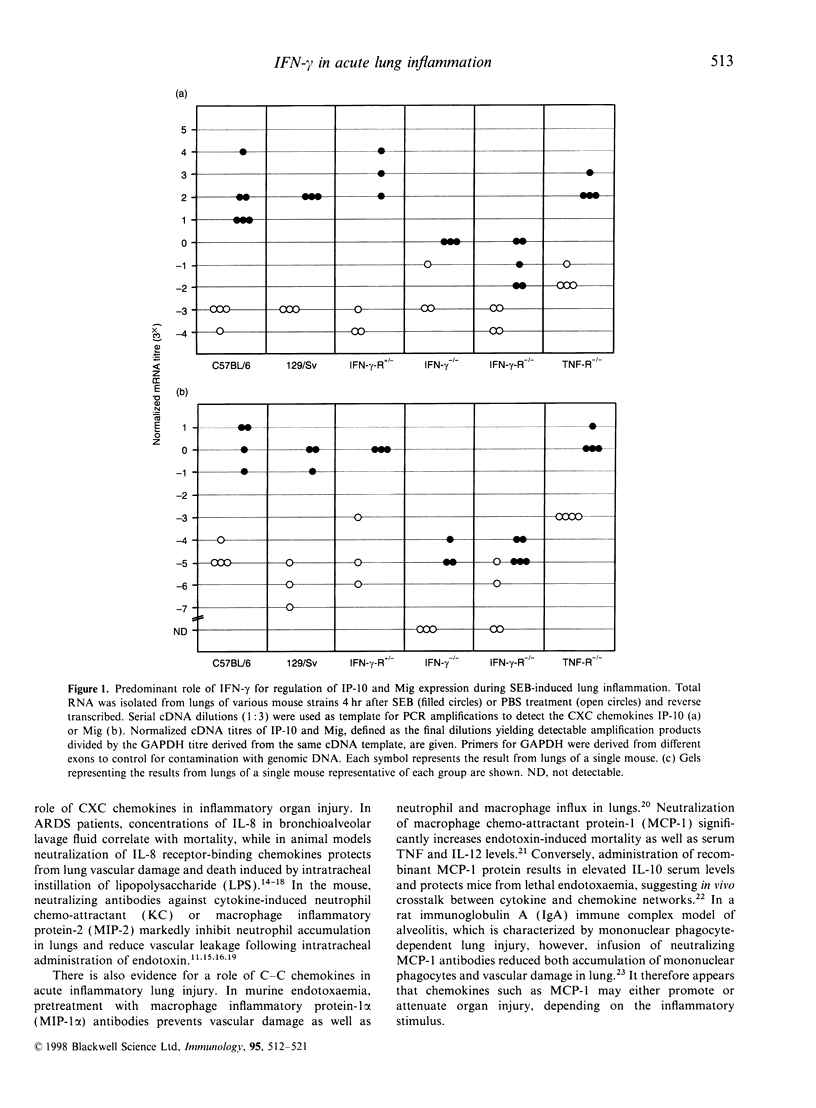
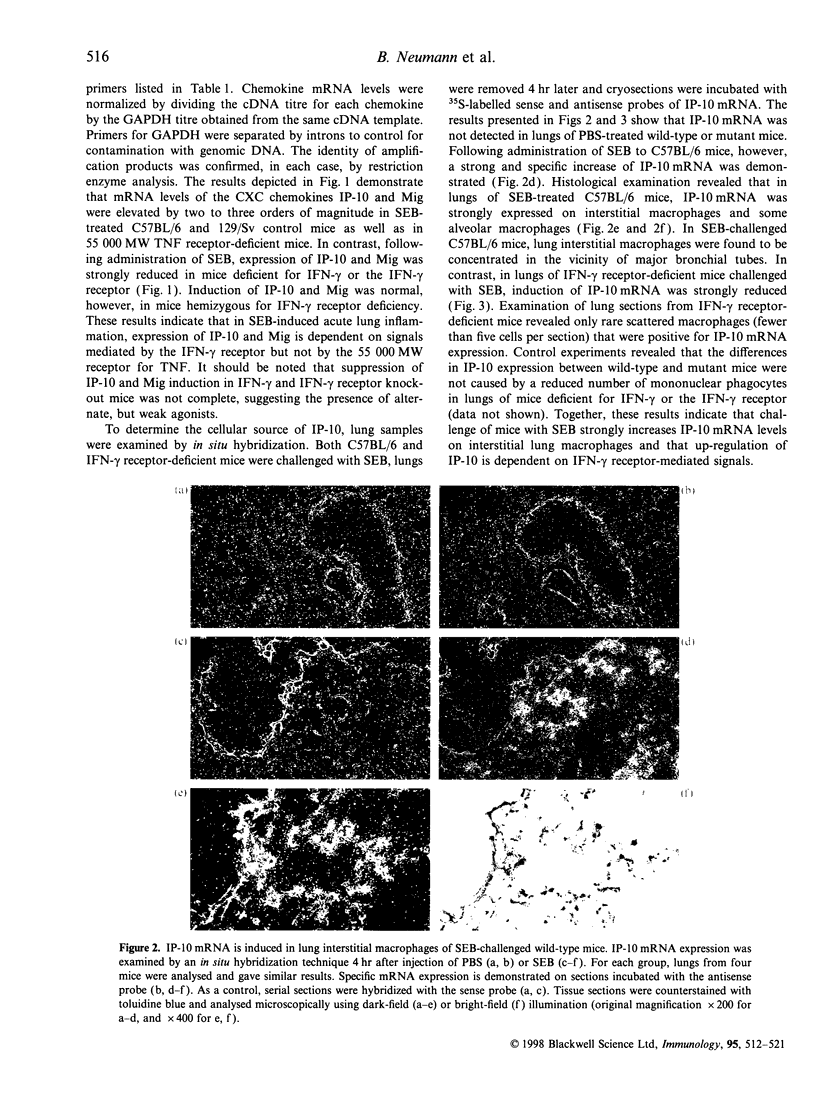
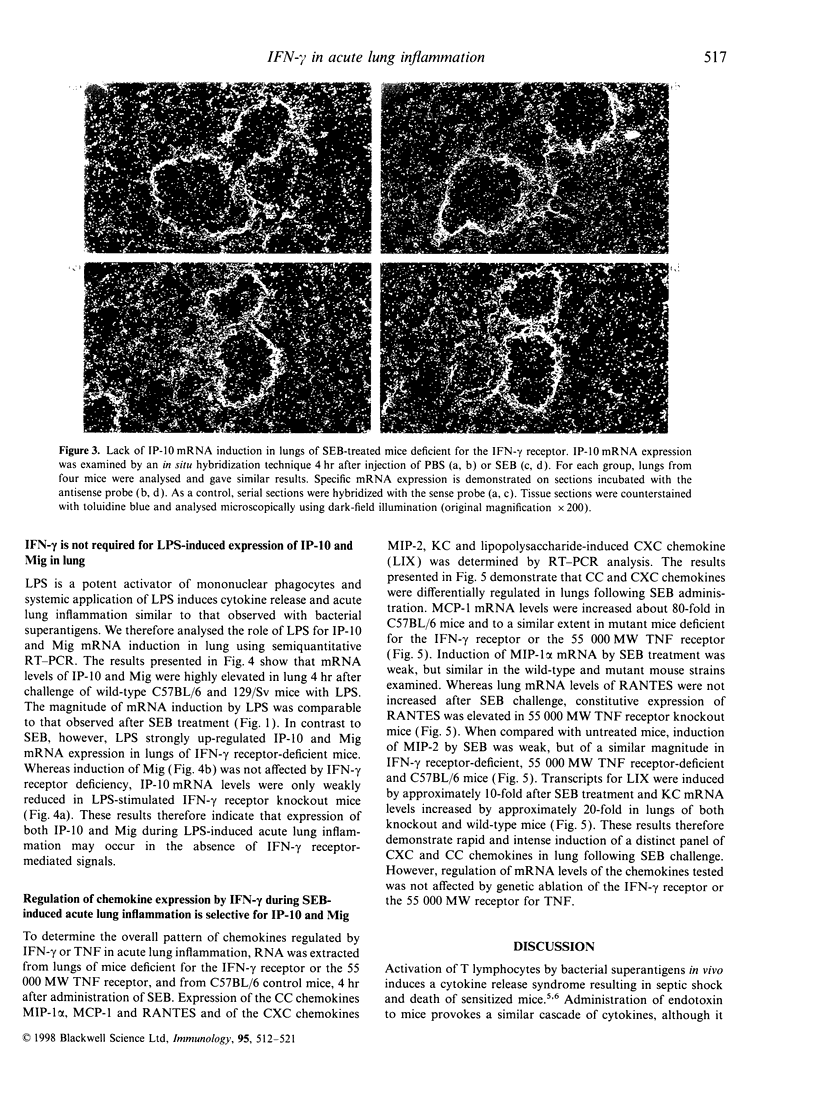
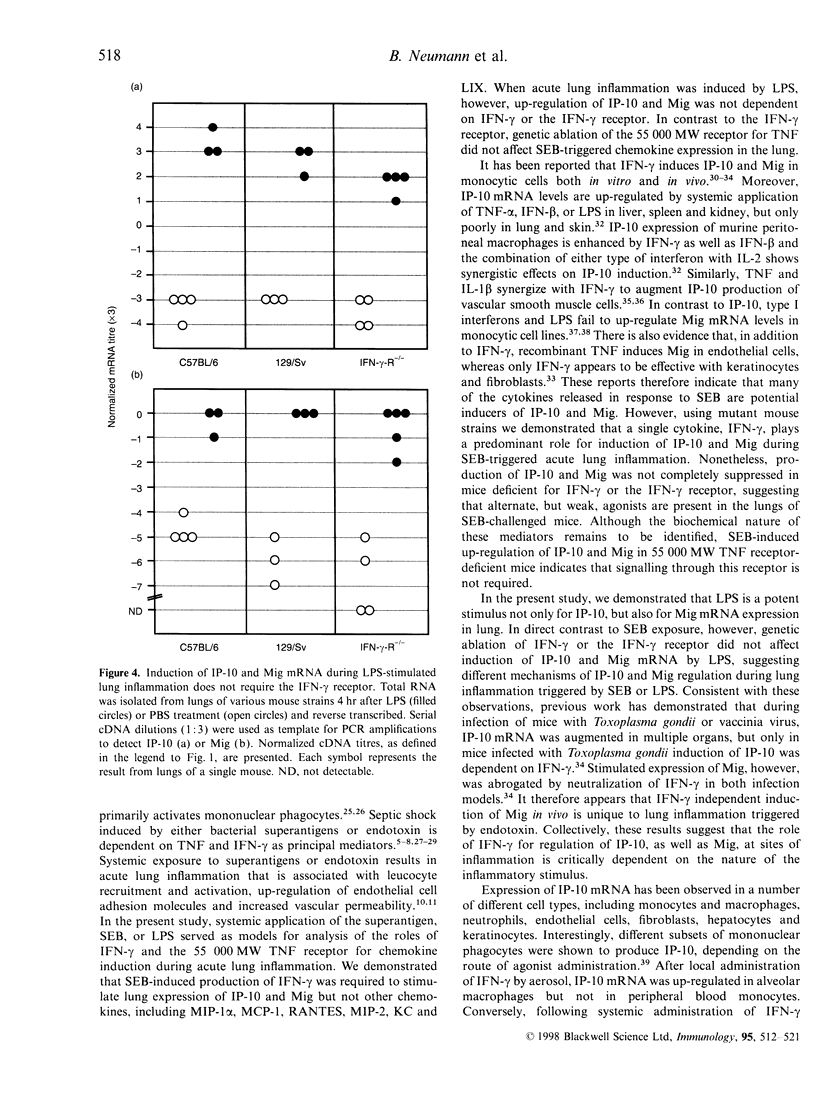
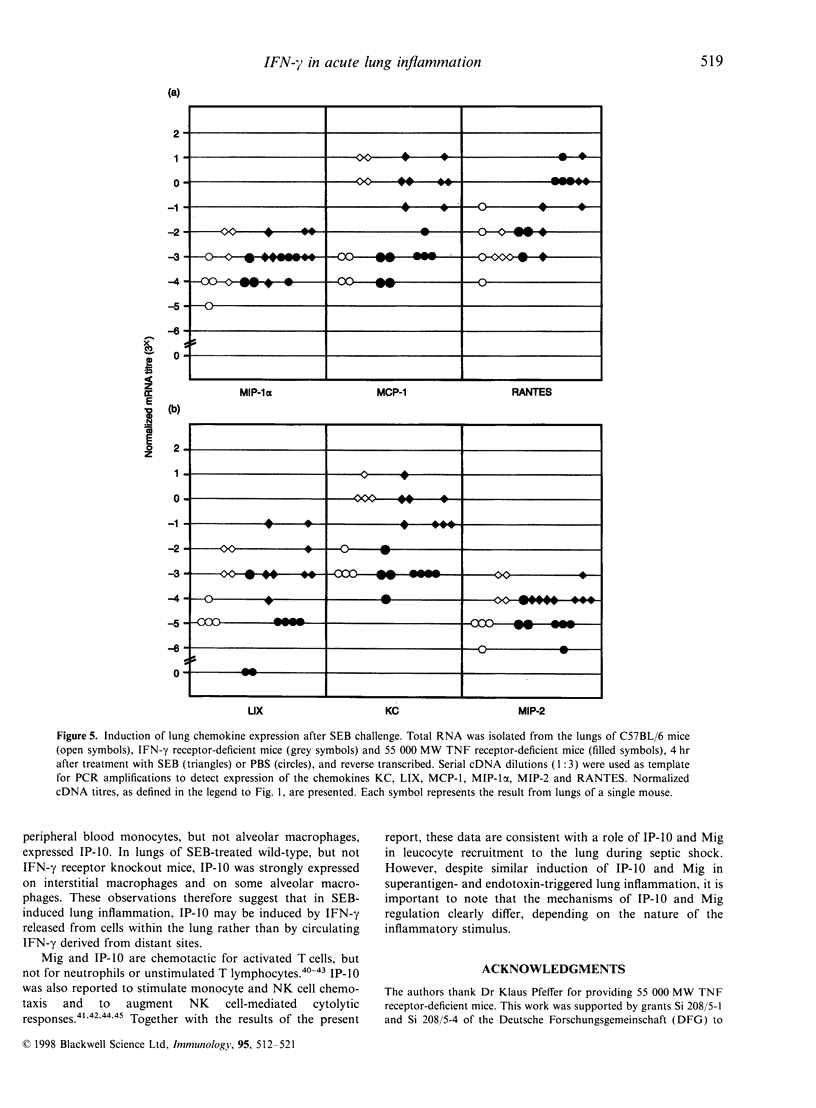
Challenge of the immune system with bacterial superantigens or endotoxin induces the systemic release of cytokines followed by lethal septic shock. The lung is particularly susceptible to systemic toxin exposure resulting in acute leucocyte infiltration and vascular damage. In the present study, the functions of interferon-gamma (IFN-gamma) and tumour necrosis factor (TNF) for chemokine regulation during acute lung inflammation were examined. Following administration of the superantigen, staphylococcal enterotoxin B (SEB), lung mRNA levels of the chemokines cytokine-induced neutrophil chemo-attractant (KC), lipopolysaccharide-induced CXC chemokine (LIX), macrophage chemotactic protein-1 (MCP-1), macrophage inflammatory protein (MIP)-1alpha and MIP-2 were increased to a similar extent both in controls and in mice deficient for the IFN-gamma or 55 000 MW TNF receptors. In contrast, interferon-inducible protein-10 (IP-10) and monokine induced by IFN-gamma (Mig) mRNA expression was markedly reduced in mice deficient for IFN-gamma or IFN-gamma receptor, but not in 55 000 MW TNF receptor knockout mice. In situ hybridization experiments demonstrated that IP-10 was highly expressed in lung interstitial macrophages of C57BL/6, but not of IFN-gamma receptor-deficient mice. In contrast to SEB administration, treatment with lipopolysaccharide resulted in a strong induction of IP-10 and Mig in IFN-gamma receptor-deficient mice. Together, these results establish a critical function of IFN-gamma for chemokine induction in acute lung inflammation that is dependent on the nature of the inflammatory stimulus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amichay D., Gazzinelli R. T., Karupiah G., Moench T. R., Sher A., Farber J. M. Genes for chemokines MuMig and Crg-2 are induced in protozoan and viral infections in response to IFN-gamma with patterns of tissue expression that suggest nonredundant roles in vivo. J Immunol. 1996 Nov 15;157(10):4511–4520. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis. 1986 May;133(5):913–927. [PubMed] [Google Scholar]
- Broaddus V. C., Boylan A. M., Hoeffel J. M., Kim K. J., Sadick M., Chuntharapai A., Hébert C. A. Neutralization of IL-8 inhibits neutrophil influx in a rabbit model of endotoxin-induced pleurisy. J Immunol. 1994 Mar 15;152(6):2960–2967. [PubMed] [Google Scholar]
- Car B. D., Eng V. M., Schnyder B., Ozmen L., Huang S., Gallay P., Heumann D., Aguet M., Ryffel B. Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994 May 1;179(5):1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrendele H. C., Estess P., Siegelman M. H. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997 Oct 24;278(5338):672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- Farber J. M. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5238–5242. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M. HuMig: a new human member of the chemokine family of cytokines. Biochem Biophys Res Commun. 1993 Apr 15;192(1):223–230. doi: 10.1006/bbrc.1993.1403. [DOI] [PubMed] [Google Scholar]
- Farber J. M. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997 Mar;61(3):246–257. [PubMed] [Google Scholar]
- Freudenberg M. A., Keppler D., Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986 Mar;51(3):891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert C. W., Huang S., Danaee H., Paulauskis J. D., Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol. 1995 Jan 1;154(1):335–344. [PubMed] [Google Scholar]
- Gaus H., Miethke T., Wagner H., Heeg K. Superantigen-induced anergy of V beta 8+ CD4+ T cells induces functional but non-proliferative T cells in vivo. Immunology. 1994 Nov;83(3):333–340. [PMC free article] [PubMed] [Google Scholar]
- Heeg K., Miethke T., Wagner H. Superantigen-mediated lethal shock: the functional state of ligand-reactive T cells. Curr Top Microbiol Immunol. 1996;216:83–100. doi: 10.1007/978-3-642-80186-0_4. [DOI] [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Herrmann T., MacDonald H. R. T cell recognition of superantigens. Curr Top Microbiol Immunol. 1991;174:21–38. doi: 10.1007/978-3-642-50998-8_2. [DOI] [PubMed] [Google Scholar]
- Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinkernagel R. M., Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993 Mar 19;259(5102):1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Jaffe H. A., Buhl R., Mastrangeli A., Holroyd K. J., Saltini C., Czerski D., Jaffe H. S., Kramer S., Sherwin S., Crystal R. G. Organ specific cytokine therapy. Local activation of mononuclear phagocytes by delivery of an aerosol of recombinant interferon-gamma to the human lung. J Clin Invest. 1991 Jul;88(1):297–302. doi: 10.1172/JCI115291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen P. M., van Damme J., Put W., de Jong I. W., Taylor F. B., Jr, Hack C. E. Monocyte chemotactic protein 1 is released during lethal and sublethal bacteremia in baboons. J Infect Dis. 1995 Jun;171(6):1640–1642. doi: 10.1093/infdis/171.6.1640. [DOI] [PubMed] [Google Scholar]
- Jones M. L., Mulligan M. S., Flory C. M., Ward P. A., Warren J. S. Potential role of monocyte chemoattractant protein 1/JE in monocyte/macrophage-dependent IgA immune complex alveolitis in the rat. J Immunol. 1992 Sep 15;149(6):2147–2154. [PubMed] [Google Scholar]
- Liao F., Rabin R. L., Yannelli J. R., Koniaris L. G., Vanguri P., Farber J. M. Human Mig chemokine: biochemical and functional characterization. J Exp Med. 1995 Nov 1;182(5):1301–1314. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster A. D., Unkeless J. C., Ravetch J. V. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985 Jun 20;315(6021):672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- Maghazachi A. A., Skalhegg B. S., Rolstad B., Al-Aoukaty A. Interferon-inducible protein-10 and lymphotactin induce the chemotaxis and mobilization of intracellular calcium in natural killer cells through pertussis toxin-sensitive and -insensitive heterotrimeric G-proteins. FASEB J. 1997 Aug;11(10):765–774. doi: 10.1096/fasebj.11.10.9271361. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Yokoi K., Mukaida N., Harada A., Yamashita J., Watanabe Y., Matsushima K. Pivotal role of interleukin-8 in the acute respiratory distress syndrome and cerebral reperfusion injury. J Leukoc Biol. 1997 Nov;62(5):581–587. doi: 10.1002/jlb.62.5.581. [DOI] [PubMed] [Google Scholar]
- Miethke T., Wahl C., Heeg K., Echtenacher B., Krammer P. H., Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992 Jan 1;175(1):91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miethke T., Wahl C., Regele D., Gaus H., Heeg K., Wagner H. Superantigen mediated shock: a cytokine release syndrome. Immunobiology. 1993 Nov;189(3-4):270–284. doi: 10.1016/S0171-2985(11)80362-1. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Cohen A. B., Nagao S., Griffith D., Maunder R. J., Martin T. R., Weiner-Kronish J. P., Sticherling M., Christophers E., Matthay M. A. Elevated levels of NAP-1/interleukin-8 are present in the airspaces of patients with the adult respiratory distress syndrome and are associated with increased mortality. Am Rev Respir Dis. 1992 Aug;146(2):427–432. doi: 10.1164/ajrccm/146.2.427. [DOI] [PubMed] [Google Scholar]
- Narumi S., Finke J. H., Hamilton T. A. Interferon gamma and interleukin 2 synergize to induce selective monokine expression in murine peritoneal macrophages. J Biol Chem. 1990 Apr 25;265(12):7036–7041. [PubMed] [Google Scholar]
- Narumi S., Wyner L. M., Stoler M. H., Tannenbaum C. S., Hamilton T. A. Tissue-specific expression of murine IP-10 mRNA following systemic treatment with interferon gamma. J Leukoc Biol. 1992 Jul;52(1):27–33. doi: 10.1002/jlb.52.1.27. [DOI] [PubMed] [Google Scholar]
- Neumann B., Engelhardt B., Wagner H., Holzmann B. Induction of acute inflammatory lung injury by staphylococcal enterotoxin B. J Immunol. 1997 Feb 15;158(4):1862–1871. [PubMed] [Google Scholar]
- Pfeffer K., Matsuyama T., Kündig T. M., Wakeham A., Kishihara K., Shahinian A., Wiegmann K., Ohashi P. S., Krönke M., Mak T. W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993 May 7;73(3):457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Rothe J., Lesslauer W., Lötscher H., Lang Y., Koebel P., Köntgen F., Althage A., Zinkernagel R., Steinmetz M., Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993 Aug 26;364(6440):798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- Schmal H., Shanley T. P., Jones M. L., Friedl H. P., Ward P. A. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J Immunol. 1996 Mar 1;156(5):1963–1972. [PubMed] [Google Scholar]
- Simms H. H., D'Amico R. Increased PMN CD11b/CD18 expression following post-traumatic ARDS. J Surg Res. 1991 Apr;50(4):362–367. doi: 10.1016/0022-4804(91)90204-y. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Lukacs N. W., Greenberger M. J., Danforth J. M., Kunkel R. G., Strieter R. M. Macrophage inflammatory protein-1 alpha mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J Immunol. 1995 Aug 1;155(3):1515–1524. [PubMed] [Google Scholar]
- Taub D. D., Lloyd A. R., Conlon K., Wang J. M., Ortaldo J. R., Harada A., Matsushima K., Kelvin D. J., Oppenheim J. J. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993 Jun 1;177(6):1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub D. D., Longo D. L., Murphy W. J. Human interferon-inducible protein-10 induces mononuclear cell infiltration in mice and promotes the migration of human T lymphocytes into the peripheral tissues and human peripheral blood lymphocytes-SCID mice. Blood. 1996 Feb 15;87(4):1423–1431. [PubMed] [Google Scholar]
- Taub D. D., Sayers T. J., Carter C. R., Ortaldo J. R. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995 Oct 15;155(8):3877–3888. [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Vanguri P., Farber J. M. Identification of CRG-2. An interferon-inducible mRNA predicted to encode a murine monokine. J Biol Chem. 1990 Sep 5;265(25):15049–15057. [PubMed] [Google Scholar]
- Wang X., Yue T. L., Ohlstein E. H., Sung C. P., Feuerstein G. Z. Interferon-inducible protein-10 involves vascular smooth muscle cell migration, proliferation, and inflammatory response. J Biol Chem. 1996 Sep 27;271(39):24286–24293. doi: 10.1074/jbc.271.39.24286. [DOI] [PubMed] [Google Scholar]
- Zisman D. A., Kunkel S. L., Strieter R. M., Tsai W. C., Bucknell K., Wilkowski J., Standiford T. J. MCP-1 protects mice in lethal endotoxemia. J Clin Invest. 1997 Jun 15;99(12):2832–2836. doi: 10.1172/JCI119475. [DOI] [PMC free article] [PubMed] [Google Scholar]