Abstract
Some leprosy patients suffer from clinical episodes associated with tissue damage which are designated as Type 1 (reversal reaction) when localized to the lesions and Type 2 (erythema nodosum leprosum, ENL) when accompanied by systemic involvement. We had reported earlier that stable, non-reaction lepromatous leprosy subjects show T helper 2 (Th2)- and Th0- but not Th1-like responses in the peripheral blood. To further understand the development of Th-like responses during disease, 32 lepromatous patients undergoing reactions were studied using cytokine-specific reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) in peripheral blood and some skin biopsies. Of interest was the evidence of a Th1-like response with presence of interferon-gamma (IFN-gamma) and absence of interleukin-4 (IL-4) mRNA in the peripheral blood mononuclear cells (PBMC) of 85 and 64% of Type 1 and 2 reaction patients, respectively, and in all reaction sites. Whereas a Th0- was seen in some, a Th2-like response was absent. IL-12p40 mRNA was seen in 21/25 ENL and all Type 1 reaction subjects irrespective of the Th phenotype. IL-12p40 and IFN-gamma were detectable in unstimulated PBMC suggesting an in vivo priming during reactions. IL-10 was mainly associated with adherent cells and showed a differential expression in the two reactions. It was present in the PBMC of ENL but not in reversal reaction patients. Moreover, it was not detectable in the skin lesions of either type of reactions. A Th1-like cytokine profile was associated with immunopathology and persisted up to 6-7 months after the onset of reactions.
Full text
PDF


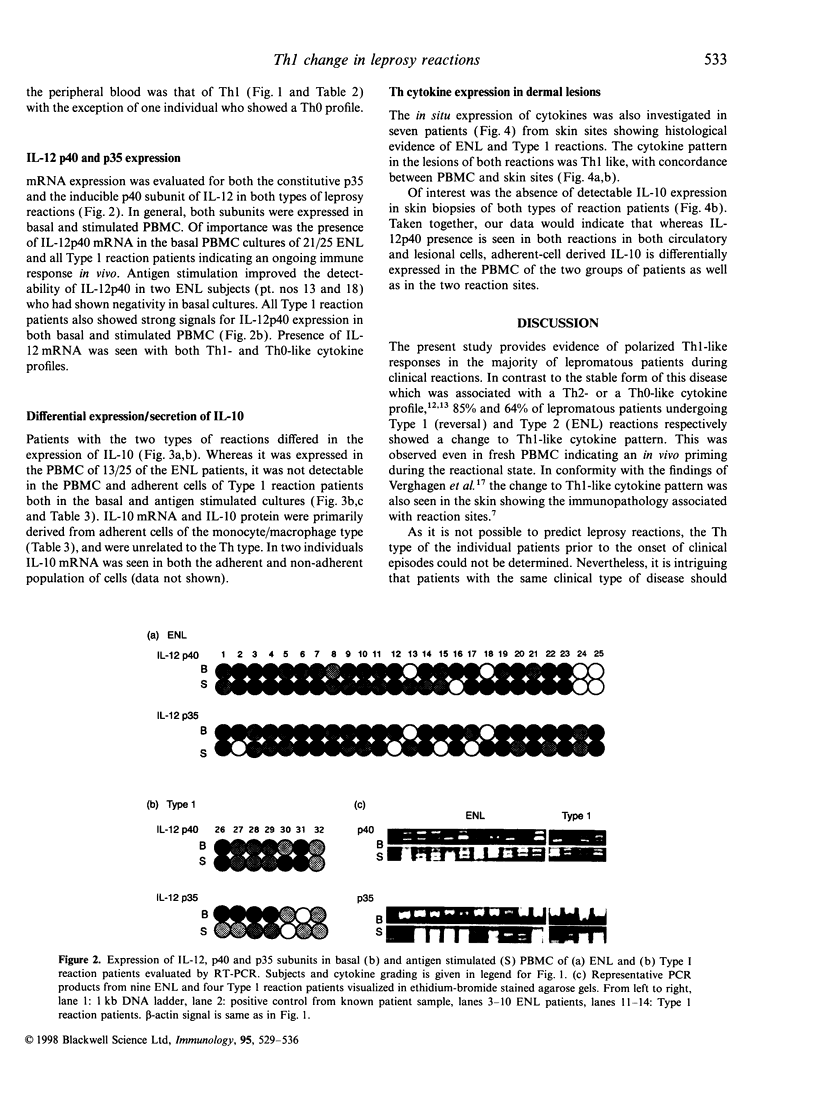



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. F., Abrams J. S., Lu S., Sieling P. A., Rea T. H., Modlin R. L. Patterns of cytokine production by mycobacterium-reactive human T-cell clones. Infect Immun. 1993 Jan;61(1):197–203. doi: 10.1128/iai.61.1.197-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Shearer G. M. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993 Mar;14(3):107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Varkila K., Scott P., Chatelain R. Role of cytokines in the differentiation of CD4+ T-cell subsets in vivo. Immunol Rev. 1991 Oct;123:189–207. doi: 10.1111/j.1600-065x.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Giudizi M. G., Biagiotti R., Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993 Jan 15;150(2):353–360. [PubMed] [Google Scholar]
- Laal S., Bhutani L. K., Nath I. Natural emergence of antigen-reactive T cells in lepromatous leprosy patients during erythema nodosum leprosum. Infect Immun. 1985 Dec;50(3):887–892. doi: 10.1128/iai.50.3.887-892.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetti R., Gerosa F., Giudizi M. G., Biagiotti R., Parronchi P., Piccinni M. P., Sampognaro S., Maggi E., Romagnani S., Trinchieri G. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J Exp Med. 1994 Apr 1;179(4):1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra N., Selvakumar M., Singh S., Bharadwaj M., Ramesh V., Misra R. S., Nath I. Monocyte derived IL 10 and PGE2 are associated with the absence of Th 1 cells and in vitro T cell suppression in lepromatous leprosy. Immunol Lett. 1995 Dec;48(2):123–128. doi: 10.1016/0165-2478(95)02455-7. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Mehra V., Jordan R., Bloom B. R., Rea T. H. In situ and in vitro characterization of the cellular immune response in erythema nodosum leprosum. J Immunol. 1986 Feb 1;136(3):883–886. [PubMed] [Google Scholar]
- Modlin R. L. Th1-Th2 paradigm: insights from leprosy. J Invest Dermatol. 1994 Jun;102(6):828–832. doi: 10.1111/1523-1747.ep12381958. [DOI] [PubMed] [Google Scholar]
- Mutis T., Kraakman E. M., Cornelisse Y. E., Haanen J. B., Spits H., De Vries R. R., Ottenhoff T. H. Analysis of cytokine production by Mycobacterium-reactive T cells. Failure to explain Mycobacterium leprae-specific nonresponsiveness of peripheral blood T cells from lepromatous leprosy patients. J Immunol. 1993 May 15;150(10):4641–4651. [PubMed] [Google Scholar]
- Narayanan R. B., Laal S., Sharma A. K., Bhutani L. K., Nath I. Differences in predominant T cell phenotypes and distribution pattern in reactional lesions of tuberculoid and lepromatous leprosy. Clin Exp Immunol. 1984 Mar;55(3):623–628. [PMC free article] [PubMed] [Google Scholar]
- O'Garra A., Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994 Jun;6(3):458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Powrie F., Menon S., Coffman R. L. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993 Nov;23(11):3043–3049. doi: 10.1002/eji.1830231147. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Sampaio E. P., Moreira A. L., Sarno E. N., Malta A. M., Kaplan G. Prolonged treatment with recombinant interferon gamma induces erythema nodosum leprosum in lepromatous leprosy patients. J Exp Med. 1992 Jun 1;175(6):1729–1737. doi: 10.1084/jem.175.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993 Apr 23;260(5107):496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- Singh S., Jenner P. J., Narayan N. P., Ramu G., Colston M. J., Prasad H. K., Nath I. Critical residues of the Mycobacterium leprae LSR recombinant protein discriminate clinical activity in erythema nodosum leprosum reactions. Infect Immun. 1994 Dec;62(12):5702–5705. doi: 10.1128/iai.62.12.5702-5705.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Wang X. H., Ohmen J. D., Uyemura K., Rea T. H., Bloom B. R., Modlin R. L. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992 Aug 15;149(4):1470–1475. [PubMed] [Google Scholar]







