Abstract
The induction and inhibition of the interferon (IFN) response and apoptosis by bovine viral diarrhea virus (BVDV) has been examined. Here we show that prior infection of cells by noncytopathogenic BVDV (ncp BVDV) fails to block transcriptional responses to alpha/beta IFN. In contrast, ncp BVDV-infected cells fail to produce IFN-α/β or MxA in response to double-stranded RNA (dsRNA) or infection with a heterologous virus (Semliki Forest virus [SFV]). ncp BVDV preinfection is unable to block cp BVDV- or SFV-induced apoptosis. The effects of ncp BVDV infection on the transcription factors controlling the IFN-β induction pathway have been analyzed. The transcription factor NF-κB was not activated following ncp BVDV infection, but ncp BVDV infection was not able to block the activation of NF-κB by either SFV or tumor necrosis factor alpha. Furthermore, ncp BVDV infection did not result in the activation of stress kinases (JNK1 and JNK2) or the phosphorylation of transcription factors ATF-2 and c-Jun; again, ncp BVDV infection was not able to block their activation by SFV. Interferon regulatory factor 3 (IRF-3) was shown to be translocated to the nuclei of infected cells in response to ncp BVDV, although DNA-binding of IRF-3 was not seen in nuclear extracts. In contrast, an IRF-3-DNA complex was observed in nuclear extracts from cells infected with SFV, but the appearance of this complex was blocked when cells were previously exposed to ncp BVDV. We conclude that the inhibition of IFN induction by this pestivirus involves a block to IRF-3 function, and we speculate that this may be a key characteristic for the survival of pestiviruses in nature.
Bovine viral diarrhea virus (BVDV) is associated with a wide variety of disorders of cattle; infection is common, but the severity of the outcome ranges from subclinical or very mild in the majority of cases to fatal mucosal disease. BVDV is a pestivirus within the family Flaviviridae, and virus strains fall into two biotypes, which differ according to their pathogenicity in certain cultured cells: one causes no visible cytopathology, while the other induces cell death through apoptosis (18, 48). Experimental infection of calves with each virus usually results in mild transient viremia and few disease signs. However, infection of a cow during pregnancy with a virus of the noncytopathogenic biotype (ncp BVDV) can result in more-pronounced effects. Abortion or teratogenic effects are common (27), but strikingly, infection during the first 120 days of pregnancy can result in the birth of persistently infected (PI) calves. Field observations together with experimental reproduction of mucosal disease have shown that cattle persistently infected with ncp BVDV develop mucosal disease after superinfection by cytopathogenic virus (cp BVDV). In contrast to strains of the ncp BVDV biotype, experimental infection of a fetus with cp BVDV does not lead to an established virus persistence (7).
Apart from the induction of cell death in infected cells, there is an additional difference between ncp BVDV and cp BVDV, in their interactions with the innate immune response: cp BVDV has been shown to induce interferon (IFN) in macrophages, whereas ncp BVDV lacks this ability (1). Importantly, infection of a fetus with cp BVDV induces a significant IFN response that is not observed following infection of a fetus with ncp BVDV (8). Evading innate responses of the host is the first step to establishing persistent infection in the absence of an acquired immune response. PI calves born after fetal infection with ncp BVDV then serve as the reservoir for acute virus infection.
Infection of cultured cells with ncp BVDV has been shown to enhance the replication of other viruses. In the case of Newcastle disease virus (NDV), a paramyxovirus which induces IFN and is sensitive to IFN, the enhancement has been associated with a reduction in the titer of IFN induced in BVDV-coinfected cultures (10). This enhancement is referred to as the END (enhancement/exaltation of NDV) effect (20) and has also been seen for an orbivirus (28). Furthermore, the activity of poly(I)·poly(C), a synthetic double-stranded RNA (dsRNA), against vesicular stomatitis virus (VSV) can be inhibited in BVDV-infected cells (30), and it has recently been shown that BVDV blocks the induction by dsRNA of IFN in bovine monocyte-derived macrophages (34). The mechanism of the BVDV block is not known: IFN induction by viruses such as NDV in fibroblastoid cell types occurs in two phases, a primary induction phase that produces IFN-β and a secondary phase that produces IFN-α (reviewed by Taniguchi et al. [39]). Since the secondary phase depends on the products of IFN-induced gene expression, BVDV could limit IFN yield by blocking either the primary induction or the secondary IFN response. The enhancement of heterologous viruses in culture is likely to be a reflection of the ability of ncp BVDV to inhibit the stimulation of innate immune responses, and it is likely that this inhibition of innate immunity accounts for the virus persistence that follows fetal infection.
In this paper we investigate the mechanism used by ncp BVDV to avoid stimulating innate immune responses in cell culture as a model of infection of the fetus. We have examined the induction of cell death, the expression of a virus infection- and IFN-induced polypeptide, MxA (19), and the induction of IFN-β in fibroblastoid cells infected by both BVDV and a model heterologous virus, Semliki Forest virus (SFV). The results show that ncp BVDV specifically targets the transcription factor IRF-3 (interferon regulatory factor 3) by preventing binding to DNA, resulting in a block to the direct virus induction of genes such as the MxA and IFN-β genes.
MATERIALS AND METHODS
Viruses and cells.
Cloned BVDV strains C874-ncp, C874-cp (13), Pe515-ncp, and Pe515-cp (6) were obtained from M. C. Clarke (Institute for Animal Health), and stocks were prepared in calf testis cell cultures (CaTe cells) maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2% fetal calf serum free of both BVDV and anti-BVDV antibody or 2% chick serum. SFV was obtained from T. F. Davison (Institute for Animal Health), and stocks were prepared in 10-day-old embryonated hens' eggs. CaTe cells were used between passages 4 and 7 and were grown in DMEM supplemented with 10% fetal calf serum free of both BVDV and anti-BVDV antibody.
Antisera.
An antiserum against human MxA was obtained from P. Staeheli, Freiburg, Germany, and used at a dilution of 1:800. A bovine hyperimmune antiserum (39/97) was obtained from a gnotobiotic calf that had been challenged with Pe515 ncp BVDV and was used at a dilution of 1:5,000. Rabbit antisera raised against a phosphospecific peptide of the activated (phosphorylated) form of stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK) and the phosphorylated forms of ATF-2 and c-Jun transcription factors were purchased from New England Biolabs UK (Hitchin, United Kingdom) and used at 1:1,000. A rabbit antiserum directed against IRF-3 (29) was kindly given by Lorena Navarro (University of California, San Francisco). The rabbit antiserum against IRF-2 has been described previously (45), and rabbit antisera against IRF-1 and p48 (IRF-9) were prepared against His-tagged and purified recombinant proteins (P. King and S. Goodbourn, unpublished data). Mouse monoclonal anti-poly(ADP-ribose) polymerase (PARP) was kindly given by G. Poirier (Quebec, Canada). An anti-rabbit-horseradish peroxidase (HRP) conjugate (used at 1:2,500), an anti-bovine-HRP conjugate (used at 1:5,000), and a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit serum were obtained from Sigma-Aldrich (Poole, United Kingdom). An ALEXA-488-conjugated goat-anti rabbit serum was obtained from Molecular Probes (Eugene, Oreg.)
BVDV infection of CaTe cells. (i) Infection with ncp BVDV.
CaTe cells at an appropriate confluency were inoculated with ncp BVDV at a multiplicity of infection (MOI) of approximately 1 to 3 PFU per cell. Infection of cells was carried out for 1 h at 37oC, after which the inoculum was removed and replaced with warm DMEM containing 2% fetal calf serum or 2% chick serum for the relevant time. Mock infection was carried out with DMEM and fetal calf serum or chick serum, or, in the case of immunofluorescence studies, with CaTe-conditioned DMEM with 2% chick serum. Cells at low confluency were used for immunofluorescence studies; in other cases, cells were used when confluent.
(ii) Infection with cp BVDV.
CaTe cells or CaTe cells infected with ncp BVDV for 48 h were infected at 2 to 3 PFU/ml with cp BVDV prepared in CaTe cells for 1 h at 37oC. At appropriate times thereafter, these cells were analyzed for MxA expression and NF-κB induction.
Plaque formation by SFV and infection of cells with SFV.
For superinfection studies, mock-infected cells or cells infected with ncp BVDV were maintained at 37oC for 48 h. For assay of SFV plaque development, ncp BVDV-infected and control CaTe cells in 6-well plates were infected with serial dilutions of SFV and overlaid with 1% agarose in maintenance medium (DMEM supplemented with 2% fetal calf serum). After various times at 37°C, the cells were stained with 0.1% toluidine blue. Plaque size was measured by using a magnifying graticule.
For assay of MxA expression, mock-infected or ncp BVDV-infected cultures were infected with SFV at a low MOI (0.01 PFU/cell). For preparation of nuclear extracts, immunofluorescence microscopy, cleavage of PARP, activation of JNK, and RNA extraction, cells were infected with SFV at a high MOI (∼10 PFU/cell).
Titration of poly(I)·poly(C) and IFN in mock-treated and ncp BVDV-infected CaTe cells.
Confluent monolayers of CaTe cells in 24-well plates were mock treated with CaTe-conditioned medium (DMEM with 2% chick serum) or infected with ncp BVDV (from CaTe cells in DMEM with 2% chick serum), strain C874, at a high MOI (1 to 2 PFU per cell). After 48 h at 37°C, the medium was removed and replaced with fresh maintenance medium containing either poly(I)·poly(C) (at 13 concentrations between 1.0 ng/ml and 10 μg/ml: 1, 3.3, 6.6, 10, 33, 66, 100, 333, 666, 1,000, 3,300, 6,600, and 10,000 ng/ml) or recombinant bovine IFN-α1 (Novartis, Basel, Switzerland) (at 12 concentrations between 0.01 and 1,000 IU/ml: 0.01, 0.033, 0.066, 0.1, 0.33, 1, 3.3, 10, 33, 100, 333, and 1,000 IU/ml). After 20 h at 37°C, cells were washed in phosphate-buffered saline (PBS) and then processed by immunoblotting for MxA protein by using an anti-MxA antiserum, followed by an anti-rabbit-HRP conjugate.
Immunoblotting.
Cultured cells were treated with 8 M urea-10 mM Tris-HCl (pH 6.8)-2% sodium dodecyl sulfate-2% 2-mercaptoethanol, subjected to electrophoresis on a 10% polyacrylamide gel, and then transferred to a polyvinylidene difluoride membrane (Amersham Pharmacia Biotech). The membrane was blocked with 5% skim milk in PBS plus 0.1% Tween 20 (PBS-T) and was then incubated in the primary antibody, usually diluted in PBS-T containing 5% skim milk at room temperature for 1 h, or according to the manufacturer's instructions. After four washes in PBS-T, the membrane was incubated in the secondary antibody for 1 h. The membrane was washed four times in PBS-T and then incubated with an enhanced chemiluminescence detection reagent (ECL; Amersham Pharmacia Biotech) according to the manufacturer's instructions and exposed to film.
IFN-α/β titration.
IFN-α/β responses in CaTe cells were determined by an MxA/chloramphenicol acetyltransferase (CAT) reporter gene assay as previously described (14). In brief, 106 MDBK-t2 cells were seeded into 6-well plates and cultured in 2 ml of medium. After overnight incubation, the medium was replaced with 1 ml of the test sample diluted 1:5 in medium containing 2% fetal calf serum. Virus was inactivated in test samples by heating at 56°C for 30 min. The heated samples were run in parallel with standard amounts (0.125 to 250 IU/ml) of recombinant bovine IFN-α1. All samples were set up in duplicate and cultured for an additional 24 h at 37°C. CAT expression in cell lysates was then determined by using a commercial enzyme-linked immunosorbent assay kit (Roche Molecular Biochemicals: Mannheim, Germany), and the values were used to determine IFN-α/β levels.
RNA isolation and RNase protection.
Total cellular RNA was prepared from 9-cm plastic petri dishes of confluent cultures of CaTe cells by using the acid phenol method and was analyzed by RNase protection as described previously (49) using a probe for bovine IFN-β1 that cross-reacts with all three known bovine IFN-β transcripts (corresponding to nucleotides 37 to 270 of the bovine IFN-β coding sequences) and a mouse γ-actin probe that protects transcripts from all mammalian species examined (12).
Electrophoretic mobility shift assays (EMSAs).
Nuclear extracts were prepared as previously described (45) from 9-cm dishes of confluent CaTe cells. Protein concentrations were determined by a Bradford assay (Bio-Rad), and 10-μg aliquots of extracts were assayed by using the PRD II element from the human IFN-β promoter as a probe for NF-κB, as described by Visvanathan and Goodbourn (41), or by using the ISG15 promoter as a probe for IRF binding, as described by Wathelet et al. (43). To identify the components of individual complexes, standard reaction mixtures were pretreated with specific antisera for 1 h at 4°C before the addition of probe.
Immunofluorescent staining.
Monolayers of CaTe cells were grown on coverslips; when they reached approximately 25% confluency, they were either mock treated or infected with ncp BVDV C874 (2 to 3 PFU/cell). After incubation at 37°C, cells were washed once with PBS and were mock infected or infected with SFV (2 to 3 PFU/cell) or cp BVDV C874 (2 to 3 PFU/cell). The superinfecting virus was allowed to adsorb for 1 h at 37°C, after which the inocula were replaced by maintenance medium and the cultures were incubated for a further 6 to 7 h (SFV) or 24 h (cp BVDV).
Cells on coverslips were rinsed once with PBS and once with PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] buffer (100 mM PIPES [pH 6.8], 2 mM MgCl2, 2 mM EGTA) and then were fixed for 30 min with 4% paraformaldehyde in PBS, followed by treatment in 0.5% Triton X-100 in PBS for 10 min. Cells were washed three times with PBS and then incubated in blocking buffer (5% normal goat serum in PBS) for 30 min at ambient temperature. Cells were incubated overnight at 4°C with a primary antibody diluted in blocking buffer, with gentle agitation, and then rinsed four times in PBS. This was followed by incubation with a fluorescent secondary-antibody conjugate (FITC conjugated for standard immunofluorescence; ALEXA-488 conjugated for confocal microscopy), diluted in blocking buffer, for 1 h at ambient temperature. Coverslips were rinsed four times in PBS and then mounted in DABCO mounting medium (2.5% DABCO and 80% glycerol in PBS) for viewing under either a conventional fluorescent microscope or a confocal microscope.
RESULTS
Enhancement of plaque formation by ncp BVDV in CaTe cells.
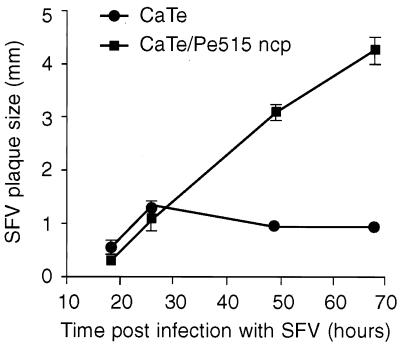
In cultured cells, ncp BVDV enhances the formation of virus plaques induced by NDV (20) and orbiviruses (28). We have tested whether this effect also occurs with SFV by using CaTe cells (passages 4 to 7). Parallel cultures of CaTe cells were set up and either mock infected or infected with ncp BVDV for 48 h; both were subsequently infected with SFV. Cultures were fixed at time points up to 3 days after SFV infection, and the sizes of well-separated plaques were measured (Fig. 1). Plaques were first seen at 18 h after infection by SFV, but at 24 h no difference in SFV plaque formation was observed between ncp BVDV-infected and control cultures. However, an increase in the size of SFV plaques in ncp BVDV-infected CaTe cells was apparent on the second and third days of incubation relative to the control cultures. Additionally, it appeared that plaque enhancement occurred only after the plaques reached a diameter of 1.5 mm. In unenhanced cultures, SFV plaques failed to increase in size beyond this point. The conclusion from these results was that SFV behaves like NDV in terms of the classical END effect.
FIG. 1.
Enhancement of SFV plaque formation by ncp BVDV. CaTe cells were either mock infected or infected with ncp BVDV (1 to 2 PFU/cell) and subsequently infected with dilutions of SFV. Cultures were fixed and stained between 18 and 66 h, and the sizes of SFV-induced plaques were measured. The figure shows the average size ± standard error of the mean of 40 to 50 plaques under each experimental condition in two independent experiments.
Kinetics of apoptosis induced by SFV in ncp BVDV-infected CaTe cells.
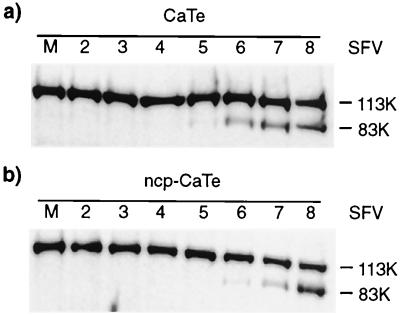
Although the initial rate of SFV plaque growth appeared to be the same for both control and ncp BVDV-infected CaTe cells, we sought to characterize this further by examining the kinetics of SFV-induced apoptosis. To ensure synchronous infection of all cells, ncp BVDV-infected and control CaTe cells were exposed to SFV at a high MOI (10 PFU per cell) and extracts were prepared at intervals up to 8 h postinfection, by which time extensive cell death was observed. The cleavage of PARP was examined to serve as a sensitive marker of apoptosis. The results of immunoblot analysis using an antibody against PARP (11) are shown in Fig. 2. In both sets of cultures the 83-kDa caspase cleavage product of PARP was first observed late in the infectious cycle of SFV, at 4 to 5 h after infection. This experiment demonstrated that preinfection of CaTe cells with ncp BVDV does not alter the kinetics of apoptosis induced by SFV.
FIG. 2.
ncp BVDV does not affect induction of apoptosis by SFV. CaTe cells were either infected with ncp BVDV (1 to 2 PFU/cell) or left uninfected and were subsequently infected with SFV at ∼10 PFU/cell. Cells were harvested in sample buffer at hourly intervals from 2 to 8 h postinfection and were subjected to immunoblotting using an anti-PARP monoclonal antibody (11). (a) CaTe cells; (b) CaTe cells previously infected with ncp BVDV. M, mock-infected cells.
Induction of IFN-stimulated gene expression in ncp BVDV-infected CaTe cells.
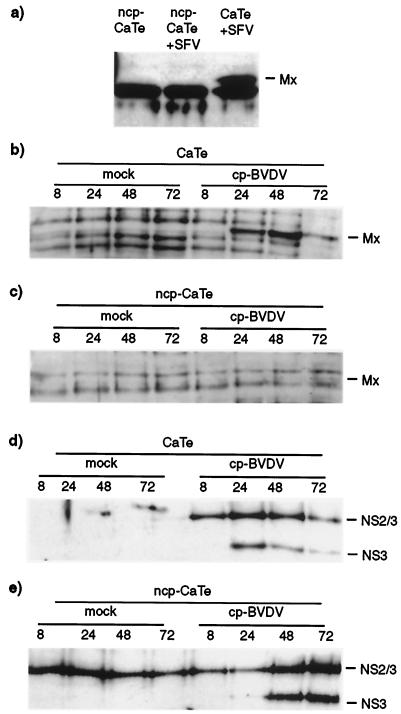
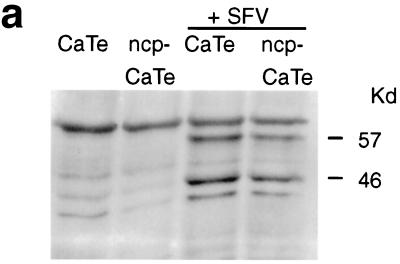
The observation that SFV infection of CaTe monolayers gave rise to larger plaques if cells had been preinfected with ncp BVDV parallels the END effect. The enhancement of NDV replication has been associated with the IFN system (28), which may indicate that ncp BVDV can interfere with the innate antiviral activities of cells. To test whether the cellular antiviral response was compromised in ncp BVDV-infected cells, we have examined induction of the MxA protein, a cellular product that is known to be induced both directly by viral infection and by virus-induced IFN-α/β (19). ncp BVDV-infected and control cells were infected with SFV at approximately 0.01 PFU per cell and were incubated overnight; samples were processed for immunoblotting with an anti-MxA antiserum. The results (Fig. 3a) demonstrated that while the MxA polypeptide was clearly induced in cells infected with SFV alone, it was not induced in cells infected with ncp BVDV or with both ncp BVDV and SFV. Infection by ncp BVDV appears to prevent the initiation or elaboration of the cellular antiviral response to SFV infection.
FIG. 3.
ncp BVDV inhibition of virus induction of an IFN-stimulated gene product, MxA. (a) ncp BVDV inhibition of MxA induction by SFV in CaTe cells. Cells were either mock infected or infected with ncp BVDV (1 to 2 PFU/cell) for 48 h. ncp BVDV-infected and mock-infected cultures were subsequently infected with SFV (0.01 PFU/cell) and incubated for 18 h. Cells were harvested in sample buffer and subjected to immunoblot analysis with a rabbit antiserum against human MxA protein. (b through e) Time course of induction of MxA and expression of viral NS3 and NS2/3 by cp BVDV in ncp BVDV-infected CaTe cells. CaTe cells were either mock infected for 48 h (b and d) or infected with ncp BVDV (1 to 2 PFU/cell) for 48 h (c and e). Cells were then either mock treated or infected with cp BVDV (2 to 3 PFU/cell). At the times indicated, cells were harvested in sample buffer and subjected to immunoblotting using either an antiserum against human MxA (b and c) or a bovine antiserum against BVDV proteins (d and e). MxA (a and b) or its expected position (c) is indicated. NS2/3 and NS3 (d and e) are BVDV polypeptides.
We next examined whether ncp BVDV could similarly block the ability of cp BVDV strains of to induce MxA in CaTe cells, since cp BVDV has been reported to induce IFN-α/β (1, 8, 34). CaTe cells were either mock infected or infected with ncp BVDV, incubated for 2 days, and then infected with cp BVDV (nominally 2 to 3 PFU per cell); after 24, 48, and 72 h they were examined for expression of cp BVDV-specific antigen (NS3) and MxA by Western blot analysis. The results showed that cp BVDV induced the synthesis of MxA protein from 24 h postinfection (Fig. 3b), but MxA was not detected in cells previously infected with ncp BVDV (Fig. 3c). We confirmed that cp BVDV was able to infect cells previously infected with ncp BVDV by detecting the cp BVDV-specific polypeptide NS3 at 48 h postinfection and later (Fig. 3d and e), and observing cytopathic effect (data not shown). Figure 3e provides confirmation that the cultures were infected with ncp BVDV.
Titration of dsRNA and IFN induction of MxA in ncp BVDV-infected cells.
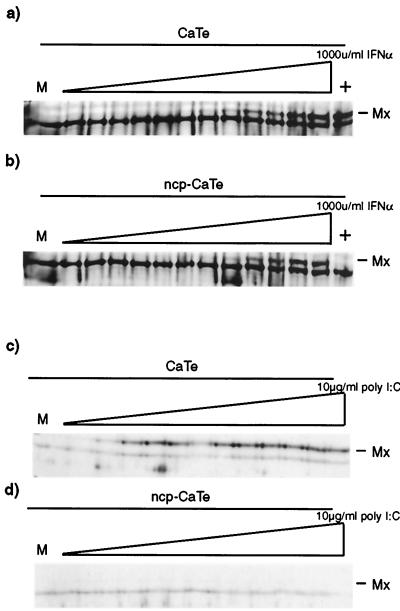
The inhibition of virus induction of MxA by ncp BVDV may have been caused by blocking either initiation of an antiviral response (e.g., by its direct induction by virus dsRNA) or its amplification by autocrine IFN production. To distinguish between these possibilities, MxA induction was measured in ncp BVDV-infected CaTe cells and compared with that in uninfected cells. Cells were treated with a range of concentrations of recombinant bovine IFN or poly(I)·poly(C), and MxA expression was measured by Western blotting. The results (Fig. 4) showed that MxA accumulated equally in mock-infected and ncp BVDV-infected cells treated with IFN at concentrations greater than or equal to 10 IU per ml added per culture. In contrast, addition of dsRNA to the medium induced MxA in uninfected cells at all concentrations from 10 ng/ml to 10 μg/ml, but MxA induction was not seen in ncp BVDV-infected cells, even at the highest concentration tested. We concluded that it is likely the induction of an antiviral response that is repressed in ncp BVDV-infected cells, since IFN signaling to stimulate the biosynthesis of MxA was not compromised at any concentration of IFN used in ncp BVDV-infected cells.
FIG. 4.
Effect of ncp BVDV infection on induction of MxA by dsRNA or by IFN-α in CaTe cells. CaTe cells were either mock infected (a and c) or infected at a high MOI with ncp BVDV (b and d) for 48 h. Cells were then treated for 20 h with fresh medium containing either various concentrations of recombinant bovine IFN-α1 (a and b) or the synthetic dsRNA poly(I)·poly(C) (c and d) at increasing concentrations as described in Materials and Methods. Cells were harvested in sample buffer and subjected to polyacrylamide gel electrophoresis and immunoblotting using a rabbit antiserum against human MxA. Lanes marked + in panels a and b indicate treatment with 10 μg of poly(I)·poly(C)/ml. M, mock treated. MxA (a, b, and c) or its expected position (d) is shown.
Infection of cells by ncp BVDV blocks induction of IFN-β by SFV.
We next tested directly whether ncp BVDV inhibits IFN induction by SFV in CaTe cells. The IFN titer was determined on a bovine (MDBK) cell line transfected with an IFN-α/β-responsive CAT gene (14). The results (Table 1) showed that SFV induced IFN-α/β in CaTe cells to between 65 and 289 IU/ml in the medium at 24 h, increasing to more than 300 IU/ml after 48 h. In contrast, at least a 100-fold reduction in IFN activity was detected following infection of CaTe cells previously infected with ncp BVDV. ncp BVDV induced no detectable IFN activity.
TABLE 1.
Effect of ncp BVDV on IFN-α/β induction by SFV
| Virus or cell treatmenta | Mean IFN-α/β titer (U/ml) | Range of titer (U/ml) |
|---|---|---|
| Mock infected CaTe cells | 0.0 | 0.0 |
| ncp BVDV (24 h) | 0.0 | 0.0 |
| ncp BVDV-SFV (24 h) | 1.8 | 0.8-3 |
| SFV (24 h) | 177 | 65-289 |
| ncp BVDV (48 h) | 0.0 | 0.0 |
| ncp BVDV-SFV (48 h) | 0.35 | 0.0-0.7 |
| SFV (48 h) | >300 | >300 |
CaTe cells in 6-well plates were either mock infected or infected with ncp BVDV at 1 to 2 PFU/cell and incubated for 2 days. Duplicate cultures were subsequently infected with 100 PFU of SFV, and samples of medium were taken at 24 and 48 h following infection with SFV. Following virus inactivation, samples were analyzed for IFN-α/β production by a bovine IFN-α/β Mx-CAT reporter assay (14).
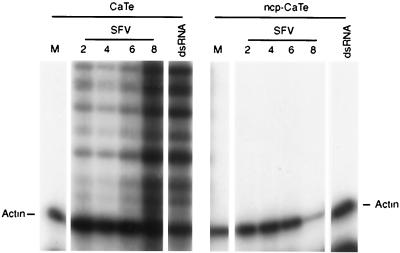
To confirm that the IFN activity contains IFN-β, we also analyzed the production of IFN-β mRNA by RNase protection assay. Cultures of CaTe cells either previously infected with ncp BVDV or untreated were infected with SFV at approximately 5 PFU per cell, and mRNA was prepared at intervals from 2 h to 8 h postinfection. Figure 5 shows that in cells infected with SFV, several IFN-β-specific protected RNAs were observed, in agreement with the fact that cattle have at least three closely related IFN-β loci (23). In contrast, no protection of the IFN-β probe was observed in cells that had been previously infected with ncp BVDV. From these experiments the conclusion was drawn that the presence of ncp BVDV can interfere with induction of IFN-β gene transcription by a heterologous virus.
FIG. 5.
ncp BVDV inhibition of IFN-β gene transcription. CaTe cells were first either mock infected or infected with ncp BVDV (1 to 2 PFU/cell) for 48 h and then either mock infected or infected with SFV (5 PFU/cell) for the times indicated, or treated with poly(I)·poly(C) (dsRNA) by addition to the medium at 100 μg/ml for 2 h. Total RNA was prepared from the cells and analyzed for the presence of IFN-β and γ-actin mRNAs by RNase protection. The actin transcript is indicated. Multiple IFN-β transcripts are detected due to the limited cross-homology to the bovine probe used and the existence of at least three bovine IFN-β genes.
Infection by ncp BVDV specifically blocks IRF-3 binding to DNA.
Since ncp BVDV blocks both IFN-β and MxA induction by SFV and cp BVDV, it seems likely that a common signaling pathway or transcription factor is targeted. Transient transfection analysis of a reporter gene under the control of an IFN-β promoter demonstrates that the block is indeed operating at the level of transcription (data not shown). Transcriptional activation of the IFN-β promoter is well characterized and is initiated by the binding of several transcription factors (a homodimer of ATF-2 or a heterodimer of ATF-2/c-Jun, with NF-κB and IRF-3) which are activated in response to virus infection through the intermediate activity of protein kinases (43); activation of IRF-3 is also probably required for direct virus induction of MxA. ncp BVDV could block the activation, nuclear localization, or DNA binding of any or all of these transcription factors.
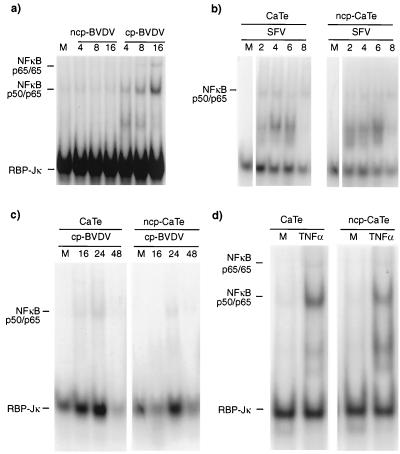
To assess the activation of NF-κB, EMSAs were carried out on nuclear extracts from CaTe cells that had previously been either mock infected or infected with either ncp BVDV or cp BVDV. ncp BVDV did not result in activation of NF-κB, but NF-κB activation was seen in cells infected by cp BVDV from 4 h after infection (Fig. 6a). To examine whether ncp BVDV could block NF-κB activation, cells were first either mock infected or infected with ncp BVDV and then infected with SFV. The results showed (Fig. 6b) that NF-κB induction by SFV is not impaired by preinfection of cells with ncp BVDV. In addition, ncp BVDV preinfection was also unable to block the induction observed with cp BVDV (Fig. 6c). These results showed that ncp BVDV infection alone was incapable of inducing NF-κB (in contrast to the properties of cp BVDV, which behaved like SFV, albeit with a reduced NF-κB induction) and did not block its activation by virus.
FIG. 6.
ncp BVDV has no effect on the induction of NF-κB. (a) CaTe cells were either mock infected (M) or infected with either ncp BVDV or cp BVDV (1 to 2 PFU/cell) for the indicated times. (b) CaTe cells were either mock infected or infected with ncp BVDV (1 to 2 PFU/cell) for 48 h and then either mock infected or infected with SFV (5 PFU/cell) for the times indicated. (c) CaTe cells were either mock infected or infected with ncp BVDV (1 to 2 PFU/cell) for 48 h and then either mock infected or infected with cp BVDV (1 to 2 PFU/cell). (d) CaTe cells were either mock infected or infected with ncp BVDV (1 to 2 PFU/cell) for 48 h and then either mock treated or treated with human TNF-α (10 ng/ml) for 90 min. Nuclear extracts were prepared from each experiment and analyzed by EMSA using the PRD II probe from the human IFN-β gene (41). This probe is able to bind NF-κB as the p65 homodimer and p50/p65 heterodimer and can also bind the unrelated transcription factor RBP-Jκ (S. Goodbourn and K. Mellits, unpublished data). The mobilities of the DNA-protein complexes are indicated at the left of each panel.
NF-κB activation can be promoted by many stimuli, including tumor necrosis factor alpha (TNF-α). It has been reported that the antiviral effects of TNF-α are inhibited in ncp BVDV-infected cultures (5, 35). To extend the observations above on NF-κB induction by viruses, TNF-α induction of NF-κB in BVDV-infected CaTe cells was examined. TNF-α added to 10 ng/ml led to NF-κB DNA binding activity in nuclear extracts of both ncp BVDV-infected and mock-infected CaTe cells (Fig. 6d). The conclusions drawn from these experiments were that NF-κB activation by a virus or TNF-α was not blocked by ncp BVDV and correlated with the dominance of virus induced apoptosis (48) rather than the apparently active inhibition of the induction of MxA.
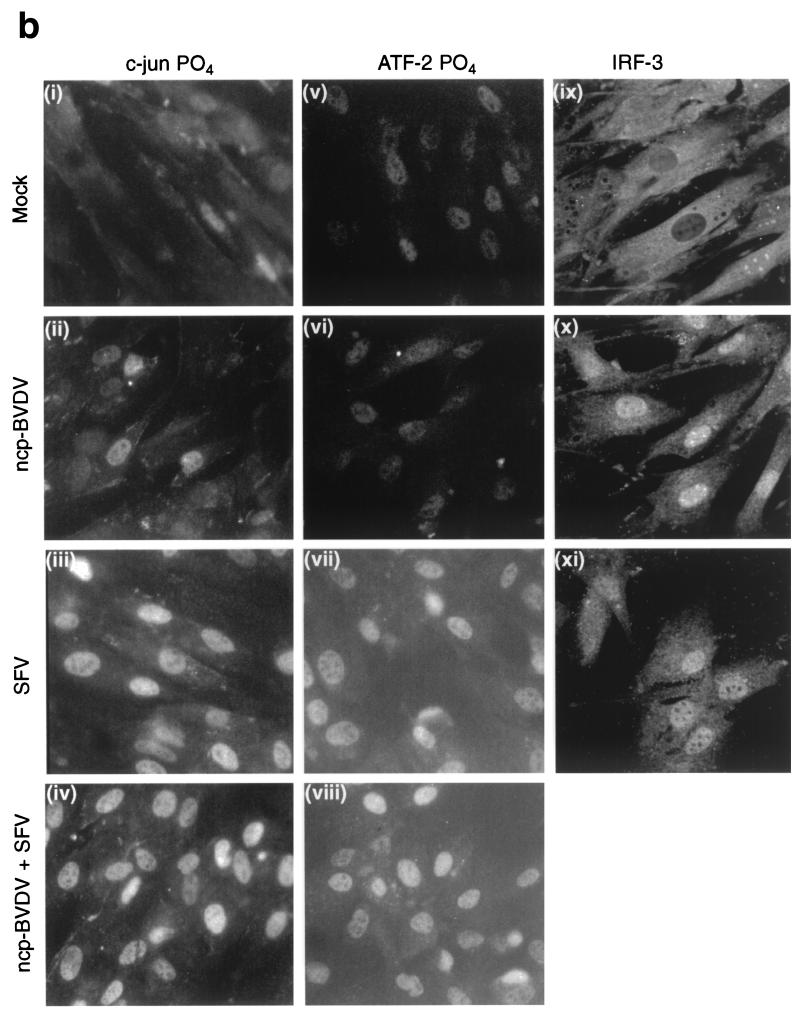
To assess the activation of c-Jun and ATF-2, the phosphorylation state of JNK was determined by Western blotting with phosphospecific antibodies. SFV stimulated the phosphorylation of JNK1 (molecular weight, 46,000 [46K]) and JNK2 (57K) in CaTe cells irrespective of their BVDV status (Fig. 7a). Similarly, no inhibition by BVDV of the phosphorylation of SEK1/MKK4-inducing kinase was observed (data not shown). Although both c-Jun and ATF-2 are constitutively nuclear, they are activated by phosphorylation; antisera specific for the phosphorylated forms of c-Jun and ATF-2 were used to examine CaTe cells infected with SFV alone or in combination with ncp BVDV. ncp BVDV infection showed no evidence for extensive phosphorylation of these factors. SFV was found to induce nuclear immunofluorescence of CaTe cells irrespective of whether or not the cells were infected with BVDV (Fig. 7b).
FIG. 7.
Activation of transcription factors in response to virus infection. (a) Effect of ncp BVDV on phosphorylation of JNK in SFV-infected CaTe cells. CaTe cells were either mock treated (first and third lanes) or infected with ncp BVDV at a high MOI (second and fourth lanes) for 48 h. Cells were then either mock treated (first and second lanes) or infected with SFV (∼10 PFU/cell) (third and fourth lanes). At 5 h postinfection, cells were harvested in sample buffer, run on a 10% minigel, and subjected to immunoblotting using a rabbit antiserum against the phosphorylated (activated) forms of JNK1 (46K) and JNK2 (57K). (b) Effects of ncp BVDV on phosphorylation and nuclear localization of IFN-β enhanceosome transcription factors. CaTe cells were either mock infected for 48 h (i, v, and ix), infected with ncp BVDV at a high MOI for 48 h (ii, vi, and x), infected with SFV at a high MOI for 6 h (iii, vii, and xi), or infected with SFV at a high MOI for 6 h in cells preinfected with ncp BVDV for 48 h (iv and viii). At these times cells were fixed, permeabilized, and then stained using either a rabbit antiserum specific for the phosphorylated form of c-Jun (i, ii, iii, and iv), an antiserum specific for the phosphorylated form of ATF-2 (v, vi, vii, and viii), or an antibody detecting IRF-3 (ix, x, and xi). For c-Jun-P and ATF-2-P, staining was revealed using a goat anti-rabbit secondary antibody conjugated to FITC and was examined using conventional fluorescence microscopy. For IRF-3, staining was revealed using a goat anti-rabbit secondary antibody conjugated to the fluorochrome ALEXA-488 and was examined using confocal microscopy.
To assess the activation of IRF-3, we carried out immunofluorescence of CaTe cells using an IRF-3 antiserum. As expected, IRF-3 was present in the cytoplasm of uninfected cells and is triggered to enter the nucleus in response to SFV infection (Fig. 7b). This also occurred following infection with both the ncp biotype of BVDV (Fig. 7b) and the cp biotype (data not shown). Thus, ncp BVDV behaves like SFV in the induction of IRF-3 translocation, which contrasts with the differences observed in the activation of c-Jun and ATF-2, MxA expression, and the induction of IFN-β synthesis.
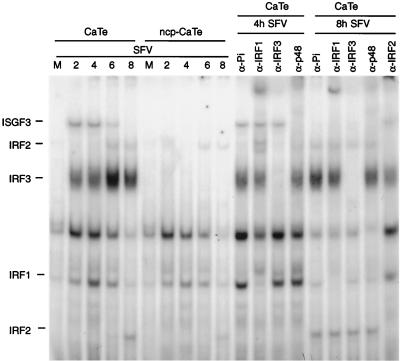
To examine the DNA binding of IFN regulatory factors (IRF), nuclear extracts were incubated with labeled DNA corresponding to the interferon-stimulated response element (ISRE) from the ISG15 promoter. Individual complexes formed on this probe were characterized with respect to their IRF components by using specific antisera against IRF-1, IRF-2, IRF-3, and IRF-9 (p48, a component of ISGF3) and a control antiserum. The results (Fig. 8) show that complexes containing IRF-1, IRF-3, and ISGF3 are formed within 2 h of SFV infection; after prolonged infection, levels of the ISGF3- and IRF-1-containing complexes decline, but this stage of infection is accompanied by the appearance of complexes containing IRF-2. Interestingly, there were no IRF-3- or ISGF3-containing complexes in nuclear extracts prepared from cells preinfected with ncp BVDV and then infected with SFV, although production of IRF-1- and IRF-2-containing complexes was unaffected. We presume that production of the IRF-3 complex precedes ISGF3 production and that the latter is produced as a consequence of IFN production in response to SFV infection, which is blocked by ncp BVDV preinfection. This interpretation is consistent with the appearance of the IRF-3 complex, but not ISGF3, after 60 min of SFV infection and the observation that ISGF3 (but not IRF-3) induction is sensitive to cycloheximide treatment (data not shown). These results show that ncp BVDV affects the assembly of DNA-IRF-3 complexes while apparently not affecting IRF-3 translocation.
FIG. 8.
Effect of ncp BVDV on assembly of SFV-induced transcription factor complexes on an ISRE probe. CaTe cells were first either mock infected or infected with ncp BVDV (1 to 2 PFU/cell) for 48 h and then either mock infected or infected with SFV (5 PFU/cell) for the times indicated. Nuclear extracts were prepared from each experiment and analyzed by EMSA using the ISRE probe from the human ISG15 gene (43). This probe is able to bind a number of members of the IRF family, as demonstrated by the nine rightmost panels, which show the supershift patterns of extracts from CaTe cells infected for 4 and 8 h with SFV incubated with antisera to individual IRFs as indicated. Pi, preimmune serum. The mobilities of complexes containing distinct IRFs are indicated to the left of the gel.
DISCUSSION
BVDV, like other pestiviruses, can infect the fetus, and if the fetus is infected during the first 3 months of pregnancy, a PI calf may be born. In this early phase of pregnancy, the capacity to generate an acquired immune response to a virus has yet to develop, and virus elimination is expected to occur through innate immunity. The survival of the virus in the fetus is therefore likely to depend on avoiding stimulation of innate immune responses, or on the virus being refractory to their action. In the cell culture system we have studied, ncp BVDV, the virus biotype that is present in PI calves, fails to induce an IFN response. By studying the effect of ncp BVDV on the induction of IFN by a heterologous virus, it is possible to gain insight into how ncp BVDV avoids the effects of innate immunity.
In this paper we have examined how ncp BVDV enhances the replication of SFV and is an example of the END effect exhibited by pestiviruses. The END effect extends to other virus families, and we have observed significant increases in plaque formation induced by coinfection with ncp BVDV and some strains of influenza viruses, encephalomyocarditis virus, and Chandipura virus (unpublished data). In no case does the presence of ncp BVDV influence the titer of heterologous virus; it solely affects plaque size. In contrast, little or no increase in plaque size was observed with bovine respiratory syncytial virus or bovine herpesvirus type 1 (data not shown). Prior infection of cells with ncp BVDV does not affect the induction of apoptosis by cp BVDV (48) or by SFV, but activation of the infection- and IFN-responsive gene MxA and IFN-β gene transcription were inhibited. Examination of the transcription factors involved in the control of IFN-β mRNA synthesis showed that IRF-3 was stimulated to migrate from the cytoplasm to the nuclei of cells infected with ncp BVDV, so we surmise that phosphorylation of IRF-3 is stimulated in ncp BVDV-infected cells. However, a later stage in binding to DNA is affected, since in cells infected by SFV an IRF-3-DNA binding complex was observed which was not present when cells had been previously infected with ncp BVDV. Consequently, we conclude that ncp BVDV affects the DNA binding properties of IRF-3. Other transcription factors proposed to be involved in the control of IFN-β synthesis that we examined (NF-κB, c-Jun, and ATF-2) were not activated in response to infection of cells by ncp BVDV, which contrasted with the response to SFV infection. Moreover, SFV activation of these transcription factors, as opposed to activation of IRF-3-DNA binding, was not inhibited by prior infection of cells with ncp BVDV.
These results illuminate previously published work on the END effect. It has been reported that IFN activation by NDV can be blocked by ncp BVDV (10) and that ncp BVDV can delay induction of IFN by orbiviruses (28). In those studies antiviral activity in culture supernatants was measured, but it was not clear whether ncp BVDV infection compromised the initiation or the elaboration of antiviral effects that were observed. We know that viruses can compromise the elaboration of IFN action: over recent years several examples of viruses that inhibit IFN signaling have been described (16). It is known that both cp and ncp BVDV are sensitive to IFN (5, 35), and it is consistent with these observations that the titration of bovine IFN on ncp BVDV-infected cultures is identical to that observed in control cultures. These results indicate that ncp BVDV cannot compromise IFN signaling and extend recently published results of studies using an antiviral assay (34). The observations presented here clearly suggest that it is solely the induction of an IFN-α/β response that is affected by ncp BVDV and not its amplification (through inhibition of IFN-α/β signal transduction).
In contrast to the sensitivity of BVDV to IFN, it has been reported that TNF-α has no antiviral effect on cp or ncp BVDV in culture (5, 35), which might suggest that TNF-α signaling is compromised in BVDV-infected cells. TNF-α signals through a series of receptors and kinases that result in the phosphorylation and proteasome degradation of the inhibitor of NF-κB, IκB, resulting in NF-κB transport to the nucleus of the cell (31). By measuring NF-κB binding activity in cells infected with ncp BVDV and treated with TNF-α, we have shown that TNF-α action through NF-κB is not inhibited by ncp BVDV. We conclude, therefore, that TNF-α signaling is not compromised by ncp BVDV.
The IRF-3-DNA complex that is induced by SFV and whose formation is blocked by ncp BVDV is likely to be reflected in other examples of the END effect. At least for NDV, IFN induction in fibroblasts is mediated through IRF-3 complexes (17, 22, 24, 25, 32, 33, 47). We do not know what other transcription factors may be present in the complex we observed; there may be other components, particularly other IRFs (2, 3, 4, 46). We have examined the IRF-3-DNA complex for the presence of IRF-7, an IRF induced following IFN treatment of cells (2), using a commercially available antiserum. The result showed no evidence for the presence of IRF-7 in the IRF-3 complex.
The ability of ncp BVDV to prevent the activation of IRF-3-DNA binding activity can explain how ncp BVDV inhibits the production, not only of IFN-β, but also of other IFN-stimulated genes. Like many other antiviral genes that are induced by IFN, MxA/Mx1 has also been shown to be induced directly by virus infection (15, 19). In the case of the ISG15 and ISG54 genes, the sequences required for direct virus activation have been shown to overlap with the ISRE required for IFN responsiveness (9, 29), and other virus-induced genes are likely to be similar. The sequences responsible for virus activation of MxA have yet to be determined, but the MxA/Mx1 promoters analyzed (mouse, human, and hen) all contain conserved ISREs. Direct virus induction of ISG15, ISG54, and RANTES gene transcription is mediated through IRF-3 (24, 29, 44), and it is likely that direct MxA activation by virus infection also proceeds by IRF-3 activation. Induction of MxA synthesis by SFV or cp BVDV may arise from one of two alternative routes: either direct virus activation or secondary amplification from IFN-β-stimulated transcription. ncp BVDV could inhibit both pathways due to the involvement of IRF-3 in virus induction of gene transcription and in the induction of IFN-β transcription, also stimulated by virus. In the case of MxA induction by SFV, we presume that it is an effect of the inhibition of IFN-β synthesis that is reflected in our results, since the experiments were carried out at a low MOI of SFV, and SFV infection at a high MOI does not lead to detectable levels of MxA protein. In contrast, induction of MxA by cp BVDV is seen in cells infected with cp BVDV at a high multiplicity. It is also noteworthy that induction of MxA is stimulated in cells infected with cp BVDV that had been subjected to gradient purification (S. J. Baigent and A. Pande, unpublished data), which is expected to reduce any effect of endogenous bovine IFN in the virus inoculum.
Activation of IRF-3 requires phosphorylation induced by a dsRNA-dependent protein kinase which is distinct from protein kinase R (PKR) (36, 37). Viruses encode inhibitors of IRF-3 activation, the best characterized of which is the influenza virus NS1 polypeptide. The ncp BVDV block to IRF-3 activation but not to NF-κB activation contrasts with the actions of the NS1 polypeptide, which blocks activation of both NF-κB and IRF-3 (38, 42). NS1 is also a dsRNA-binding protein, and this activity may block the phosphorylation of IκB and also IRF-3, either through PKR or through the putative alternative dsRNA-dependent protein kinase. It seems unlikely that ncp BVDV acts analogously to dsRNA-binding proteins.
Conspicuous are the results observed on NF-κB activation by cp BVDV infection of cells, contrasting with ncp BVDV (which failed to activate NF-κB.) We do not know how cp BVDV activates NF-κB; it may activate through PKR (40), but this needs to be established. It is clear, though, that activation by cp BVDV of NF-κB, a transcription factor involved in the control of apoptosis, does not block virus-induced apoptosis.
Recently published work on BVDV showed that ncp BVDV blocked induction of apoptosis in bovine turbinate cells transfected or treated with synthetic dsRNA and that IFN-α/β production induced by the dsRNA was inhibited in macrophages (34). Treatment of cells with synthetic dsRNA is frequently used as a model of virus infection (e.g., references 21 and 26). Our results demonstrate no repression of apoptosis induced by virus, and we adduce that inhibition by ncp BVDV of responses by the cell to dsRNA differs from that of responses to infection in this respect. Repression of the IFN response in the transfected macrophages is likely to correlate with the effects reported here on the inhibition of IRF-3 activation by ncp BVDV.
The primary induction of IFN-β mRNA synthesis is controlled by the combination of three transcription factors, only one of which, IRF-3, is affected by ncp BVDV. While ncp BVDV actively inhibits the activity of IRF-3 in DNA binding assays, we have observed no inhibitory effect of ncp BVDV on the other transcription factors that make up the IFN-β enhanceosome. How ncp BVDV effects the inhibition of IRF-3-DNA binding is the subject of further work.
To conclude, the results here implicate IRF-3-DNA binding as a factor that is blocked by ncp BVDV, which inhibits the initiation of the cellular innate antiviral and IFN responses. The lifestyle of pestiviruses is dependent on intrauterine transfer of the virus from mother to fetus prior to immune competence, which is the first step toward immunotolerance and the birth of PI calves. Infection of the fetus by ncp BVDV fails to result in the induction of IFN-α/β at the level of detection by bioassay, although ncp BVDV infection of the fetus results in a low level of induction of the IFN-stimulated gene MxA (8). Avoiding the IFN response of the fetus through inhibition of the DNA-binding activity of IRF-3 may be the critical feature that permits the survival of the virus in the fetus, the virus reservoir for many pestivirus infections, and hence survival in nature.
Acknowledgments
We are grateful to Michael Clarke, Trevor Collen, Bryan Charleston, and Ivan Morrison for advice and discussions. We thank Lorena Navarro, UCSF, and Guy Poirier, Quebec, Canada, for the gifts of antisera and Paul Monaghan for assistance with confocal microscopy. We thank Michelle Hill for excellent assistance.
REFERENCES
- 1.Adler, B., H. Adler, H. Pfister, T. W. Jungi, and E. Peterhans. 1997. Macrophages infected with cytopathic bovine viral diarrhea virus release a factor(s) capable of priming uninfected macrophages for activation-induced apoptosis. J. Virol. 71:3255-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Au, W. C., P. A. Moore, D. W. LaFleur, B. Tombal, and P. M. Pitha. 1998. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J. Biol. Chem. 273:29210-29217. [DOI] [PubMed] [Google Scholar]
- 3.Au, W. C., W. S. Yeow, and P. M. Pitha. 2001. Analysis of functional domains of interferon regulatory factor 7 and its association with IRF-3. Virology 280:273-282. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, B. J., P. A. Moore,, and P. M. Pitha. 2001. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J. Biol. Chem. 276:23382-23390. [DOI] [PubMed] [Google Scholar]
- 5.Bielefeldt-Ohmann, H., and L. A. Babiuk. 1988. Influence of interferons alpha I1 and gamma and of tumour necrosis factor on persistent infection with bovine viral diarrhoea virus in vitro. J. Gen. Virol. 69:1399-1403. [DOI] [PubMed] [Google Scholar]
- 6.Brownlie, J., M. C. Clarke, and C. J. Howard. 1984. Experimental production of fatal mucosal disease in cattle. Vet. Rec. 114:535-536. [DOI] [PubMed] [Google Scholar]
- 7.Brownlie, J., M. C. Clarke, and C. J. Howard. 1989. Experimental infection of cattle in early pregnancy with a cytopathic strain of bovine virus diarrhoea virus. Res. Vet. Sci. 46:307-311. [PubMed] [Google Scholar]
- 8.Charleston, B., M. D. Fray, S. Baigent, B. V. Carr, and W. I. Morrison. 2001. Establishment of persistent infection with non-cytopathic bovine viral diarrhoea virus in cattle is associated with a failure to induce type I interferon. J. Gen. Virol. 82:1893-1897. [DOI] [PubMed] [Google Scholar]
- 9.Daly, C., and N. C. Reich. 1995. Characterization of specific DNA-binding factors activated by double-stranded RNA as positive regulators of interferon alpha/beta-stimulated genes. J. Biol. Chem. 270:23739-23746. [DOI] [PubMed] [Google Scholar]
- 10.Diderholm, H., and Z. Dinter. 1966. Interference between strains of bovine virus diarrhea virus and their capacity to suppress interferon of a heterologous virus. Proc. Soc. Exp. Biol. Med. 121:976-980. [DOI] [PubMed] [Google Scholar]
- 11.Duriez, P. J., S. Desnoyers, J. C. Hoflack, G. M. Shah, B. Morelle, S. Bourassa, G. G. Poirier, and B. Talbot. 1997. Characterization of anti-peptide antibodies directed towards the automodification domain and apoptotic fragment of poly(ADP-ribose) polymerase. Biochim. Biophys. Acta 1334:65-72. [DOI] [PubMed] [Google Scholar]
- 12.Enoch, T., K. Zinn, and T. Maniatis. 1986. Activation of the human beta-interferon gene requires an interferon-inducible factor. Mol. Cell. Biol. 6:801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fray, M. D., M. C. Clarke, L. H. Thomas, J. W. McCauley, and B. Charleston. 1998. Prolonged nasal shedding and viraemia of cytopathogenic bovine virus diarrhoea virus in experimental late-onset mucosal disease. Vet. Rec. 143:608-611. [DOI] [PubMed] [Google Scholar]
- 14.Fray, M. D., G. E. Mann, and B. Charleston. 2001. Validation of an Mx/CAT reporter gene assay for the quantification of bovine type I interferon. J. Immunol. Methods 249:235-244. [DOI] [PubMed] [Google Scholar]
- 15.Goetschy, J. F., H. Zeller, J. Content, and M. A. Horisberger. 1989. Regulation of the interferon-inducible IFI-78K gene, the human equivalent of the murine Mx gene, by interferons, double-stranded RNA, certain cytokines, and viruses. J. Virol. 63:2616-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodbourn, S., L. J. Didcock,, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 17.Hiscott, J., P. Pitha, P. Genin, H. Nguyen, C. Heylbroeck, Y. Mamane, M. Algarte, and R. Lin. 1999. Triggering the interferon response: the role of IRF-3 transcription factor. J. Interf. Cytok. Res. 19:1-13. [DOI] [PubMed] [Google Scholar]
- 18.Hoff, H. S., and R. O. Donis. 1997. Induction of apoptosis and cleavage of poly(ADP-ribose) polymerase by cytopathic bovine viral diarrhea virus infection. Virus Res. 49:101-113. [DOI] [PubMed] [Google Scholar]
- 19.Hug, H., M. Costas, P. Staeheli, M. Aebi, and C. Weissmann. 1988. Organization of the murine Mx gene and characterization of its interferon- and virus-inducible promoter. Mol. Cell. Biol. 8:3065-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inaba, Y., Y. Tanaka, T. Kumagai, T. Omori, and H. Ito. 1968. Bovine diarrhea virus. II. END phenomenon: exaltation of Newcastle disease virus in bovine cells infected with bovine diarrhea virus. Jpn. J. Microbiol. 12:35-49. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs, B. L., and J. O. Langland. 1996. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219:339-349. [DOI] [PubMed] [Google Scholar]
- 22.Juang, Y., W. Lowther, M. Kellum, W. C. Au, R. Lin, J. Hiscott, and P. M. Pitha. 1998. Primary activation of interferon A and interferon B gene transcription by interferon regulatory factor 3. Proc. Natl. Acad. Sci. USA 95:9837-9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung, D. W., D. J. Capon, and D. V. Goeddel. 1984. The structure and bacterial expression of three distinct interferon-β genes. Bio/Technology 2:458-464. [Google Scholar]
- 24.Lin, R., C. Heylbroeck, P. Genin, P. M. Pitha, and J. Hiscott. 1999. Essential role of interferon regulatory factor 3 in direct activation of RANTES chemokine transcription. Mol. Cell. Biol. 19:959-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, R., C. Heylbroeck, P. M. Pitha, and J. Hiscott. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18:2986-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcus, P. I. 1983. Interferon induction by viruses: one molecule of dsRNA as the threshold for interferon induction. Interferon 5:115-180. [PubMed] [Google Scholar]
- 27.McGowan, M. R., and P. D. Kirkland. 1995. Early reproductive loss due to bovine pestivirus infection. Br. Vet. J. 151:263-270. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura, S., T. Shimazaki, K. Sakamoto, A. Fukusho, Y. Inoue, and N. Ogawa. 1995. Enhanced replication of orbiviruses in bovine testicle cells infected with bovine viral diarrhoea virus. J. Vet. Med. Sci. 57:677-681. [DOI] [PubMed] [Google Scholar]
- 29.Navarro, L., K. Mowen, S. Rodems, B. Weaver, N. Reich, D. Spector, and M. David. 1998. Cytomegalovirus activates interferon immediate-early response gene expression and an interferon regulatory factor 3-containing interferon-stimulated response element-binding complex. Mol. Cell. Biol. 18:3796-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi, C. R., and G. K. Kiesel. 1981. Characteristics of the polyriboinosinic acid: polyribocytidylic acid assay for noncytopathogenic bovine viral diarrhea virus. Am. J. Vet. Res. 44:1916-1919. [PubMed] [Google Scholar]
- 31.Saklatvala, J., J. Dean, and A. Finch. 1999. Protein kinase cascades in intracellular signalling by interleukin-I and tumour necrosis factor. Biochem. Soc. Symp. 64:63-77. [PubMed] [Google Scholar]
- 32.Sato, M., N. Tanaka, N. Hata, E. Oda, and T. Taniguchi. 1998. Involvement of the IRF family transcription factor IRF-3 in virus-induced activation of the IFN-β gene. FEBS Lett. 425:112-116. [DOI] [PubMed] [Google Scholar]
- 33.Schafer, S. L., R. Lin, P. A. Moore, J. Hiscott, and P. M. Pitha. 1998. Regulation of type I interferon gene expression by interferon regulatory factor-3. J. Biol. Chem. 273:2714-2720. [DOI] [PubMed] [Google Scholar]
- 34.Schweizer, M., and E. Peterhans. 2001. Noncytopathic bovine viral diarrhea virus inhibits double-stranded RNA-induced apoptosis and interferon synthesis. J. Virol. 75:4692-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sentsui, H., R. Takami, T. Nishimori, K. Murakami, T. Yokoyama, and Y. Yokomizo. 1998. Anti-viral effect of interferon-α on bovine viral diarrhea virus. J. Vet. Med. Sci. 60:1329-1333. [DOI] [PubMed] [Google Scholar]
- 36.Servant, M. J., B. ten Oever, C. LePage, L. Conti, S. Gessani, I. Julkunen, R. Lin, and J. Hiscott. 2001. Identification of distinct signaling pathways leading to the phosphorylation of interferon regulatory factor 3. J. Biol. Chem. 276:355-363. [DOI] [PubMed] [Google Scholar]
- 37.Smith, E. J., I. Marie, A. Prakash, A. Garcia-Sastre, and D. E. Levy. 2001. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or IκB kinase but is blocked by vaccinia virus E3L protein. J. Biol. Chem. 276:8951-8957. [DOI] [PubMed] [Google Scholar]
- 38.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taniguchi, T., K. Ogasawara, A. Takaoka, and N. Tanaka. 2001. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19:623-655. [DOI] [PubMed] [Google Scholar]
- 40.Vassilev, V. B., and R. O. Donis. 2000. Bovine viral diarrhea virus-induced apoptosis correlates with increased intracellular viral RNA accumulation. Virus Res. 65:95-107. [DOI] [PubMed] [Google Scholar]
- 41.Visvanathan, K. V., and S. Goodbourn. 1989. Double-stranded RNA activates binding of NF-κB to an inducible element in the human β-interferon promoter. EMBO J. 8:1129-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. Garcia-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wathelet, M. G., C. H. Lin, B. S. Parekh, L. V. Ronco, P. M. Howley, and T. Maniatis. 1998. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-β enhancer in vivo. Mol. Cell 1:507-518. [DOI] [PubMed] [Google Scholar]
- 44.Weaver, B. K., K. P. Kumar, and N. C. Reich. 1998. Interferon regulatory factor 3 and CREB-binding protein/p300 are subunits of double-stranded RNA-activated transcription factor DRAF1. Mol. Cell. Biol. 18:1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whiteside, S. T., K. V. Visvanathan, and S. Goodbourn. 1992. Identification of novel factors that bind to the PRD1 region of the human β-interferon promoter. Nucleic Acids Res. 20:1531-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeow, W. S., W. C. Au, Y. T. Juang, C. D. Fields, C. L. Dent, D. R. Gewert, and P. M. Pitha. 2000. Reconstitution of virus-mediated expression of interferon alpha genes in human fibroblast cells by ectopic interferon regulatory factor-7. J. Biol. Chem. 275:6313-6320. [DOI] [PubMed] [Google Scholar]
- 47.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, G., S. Aldridge, M. C. Clarke, and J. W. McCauley. 1996. Cell death induced by cytopathic bovine viral diarrhoea virus is mediated by apoptosis. J. Gen. Virol. 77:1677-1681. [DOI] [PubMed] [Google Scholar]
- 49.Zinn, K., D. DiMaio, and T. Maniatis. 1983. Identification of two distinct regulatory regions adjacent to the human β-interferon gene. Cell 34:865-879. [DOI] [PubMed] [Google Scholar]