Abstract
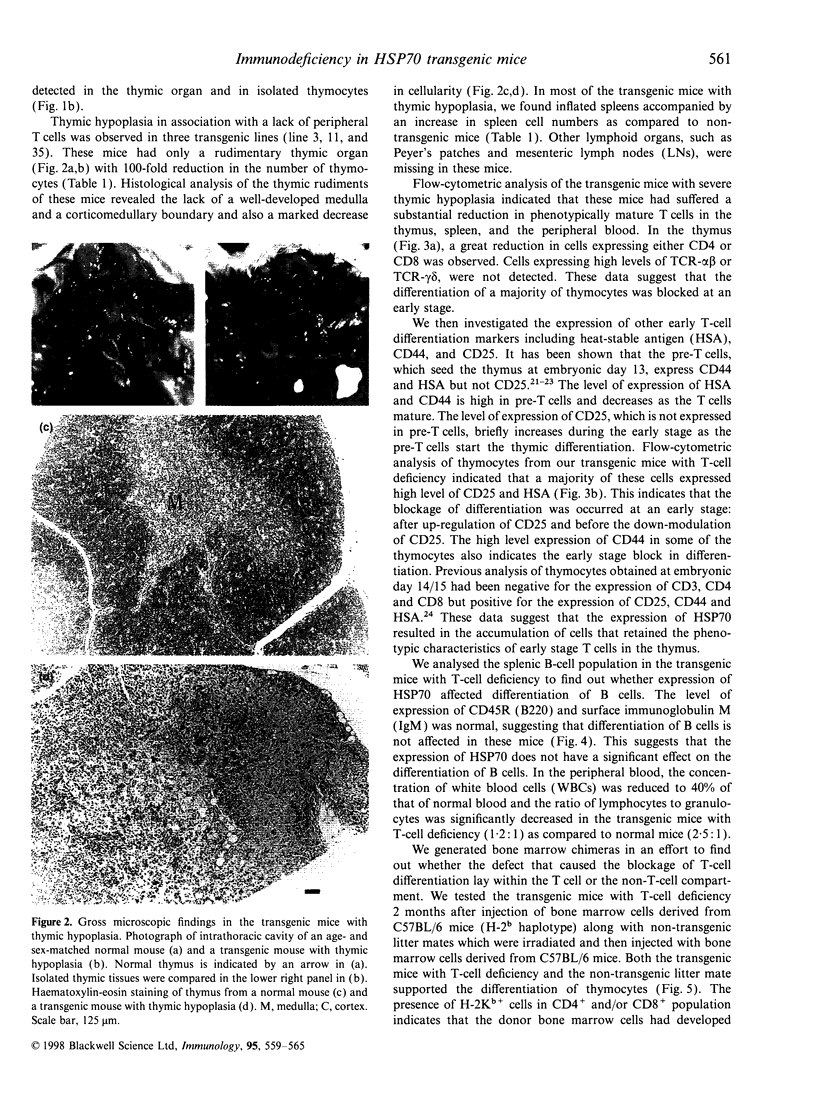
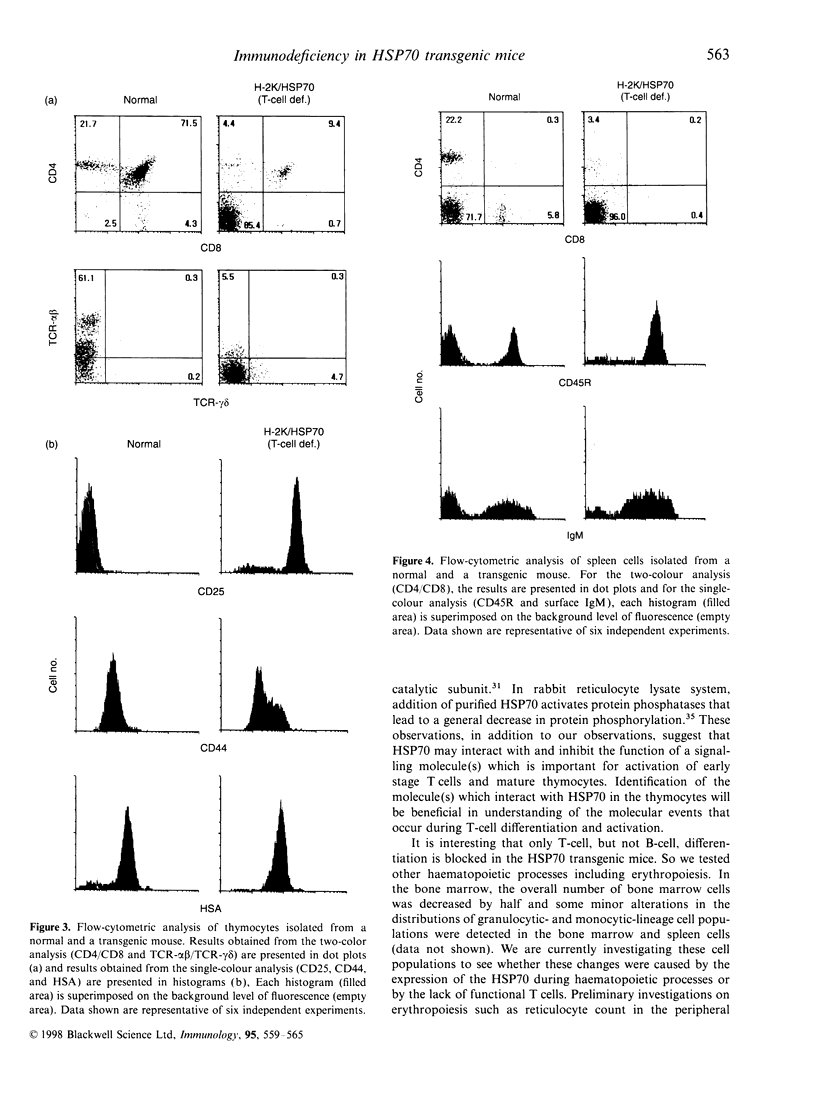
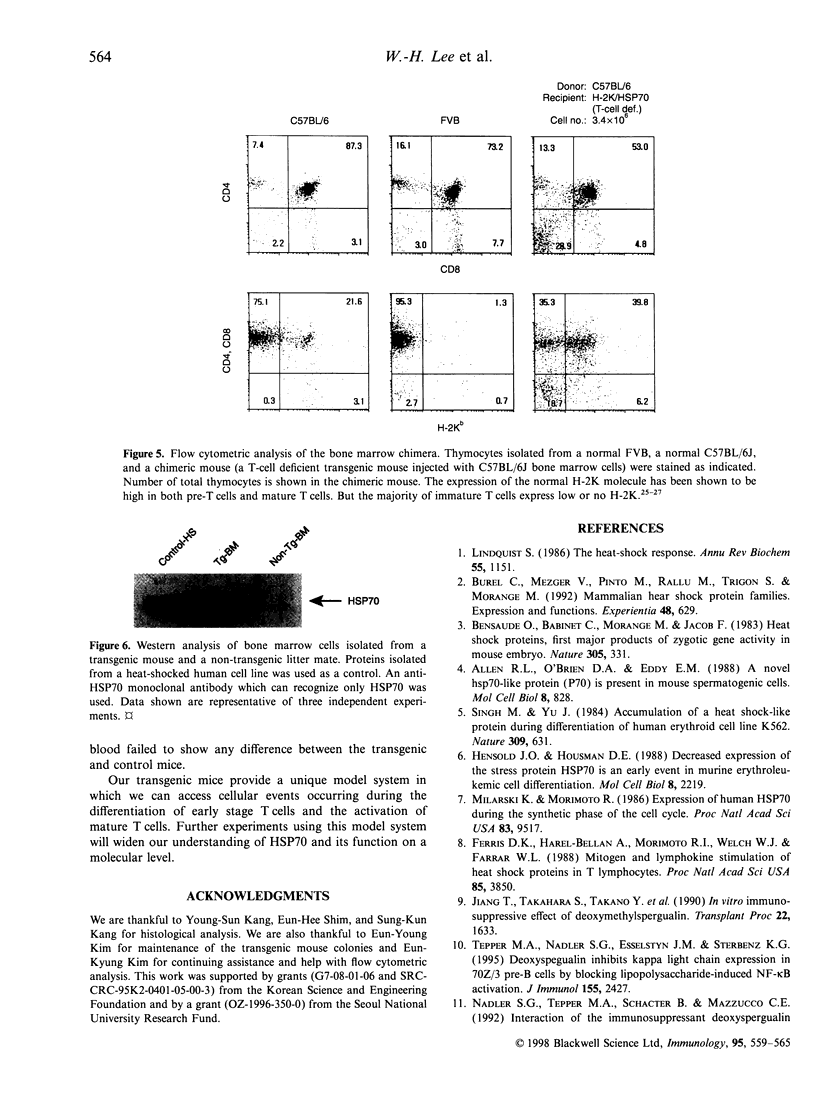
Heat shock protein 70 (HSP70) is involved not only in protein folding, but also in processes of differentiation and cell-cycle progression. Recently, HSP70 has been implicated in mediation of functions of some immunosuppressive agents. To study the role of HSP70 in differentiation of haematopoietic cells, we generated transgenic mice using the human inducible hsp70 gene fused to the mouse H-2K promoter. These mice develop a T-cell deficiency that is characterized by thymic hypoplasia and a significant reduction in peripheral T cells. The total number of thymocytes is about 100-fold less than that in normal mice. The majority of the thymocytes are immature T cells that express neither CD4 nor CD8 molecules, indicating that T cells are affected at an early stage of thymic differentiation. Expression of the transgenic HSP70 was detected both in bone marrow cells and in thymocytes. Furthermore, injection of normal bone marrow cells into the T-cell deficient mice led to the generation of mature T cells indicating that the T-cell deficiency was caused by the action of HSP70 in T cells. The blockage of differentiation occurred only in T cells, both alphabeta- and gammadelta-T-cell receptor (TCR)-bearing cells, but not in B cells, granulocytes, and monocytes. The observations suggest that HSP70 may inhibit a cellular process that is essential for the differentiation of early stage T cells. Further experiments using this model system will widen our understanding of HSP70 and its function on a molecular level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. L., O'Brien D. A., Eddy E. M. A novel hsp70-like protein (P70) is present in mouse spermatogenic cells. Mol Cell Biol. 1988 Feb;8(2):828–832. doi: 10.1128/mcb.8.2.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaude O., Babinet C., Morange M., Jacob F. Heat shock proteins, first major products of zygotic gene activity in mouse embryo. Nature. 1983 Sep 22;305(5932):331–333. doi: 10.1038/305331a0. [DOI] [PubMed] [Google Scholar]
- Burel C., Mezger V., Pinto M., Rallu M., Trigon S., Morange M. Mammalian heat shock protein families. Expression and functions. Experientia. 1992 Jul 15;48(7):629–634. doi: 10.1007/BF02118307. [DOI] [PubMed] [Google Scholar]
- Chun Y. S., Shima H., Nagasaki K., Sugimura T., Nagao M. PP1 gamma 2, a testis-specific protein-serine/threonine-phosphatase type 1 catalytic subunit, is associated with a protein having high sequence homology with the 78-kDa glucose-regulated protein, a member of the 70-kDa heat shock protein family. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3319–3323. doi: 10.1073/pnas.91.8.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayberger C., Lyu S. C., DeKruyff R., Parham P., Krensky A. M. Peptides corresponding to the CD8 and CD4 binding domains of HLA molecules block T lymphocyte immune responses in vitro. J Immunol. 1994 Aug 1;153(3):946–951. [PubMed] [Google Scholar]
- Ferris D. K., Harel-Bellan A., Morimoto R. I., Welch W. J., Farrar W. L. Mitogen and lymphokine stimulation of heat shock proteins in T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3850–3854. doi: 10.1073/pnas.85.11.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensold J. O., Housman D. E. Decreased expression of the stress protein HSP70 is an early event in murine erythroleukemic cell differentiation. Mol Cell Biol. 1988 May;8(5):2219–2223. doi: 10.1128/mcb.8.5.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husmann L. A., Shimonkevitz R. P., Crispe I. N., Bevan M. J. Thymocyte subpopulations during early fetal development in the BALB/c mouse. J Immunol. 1988 Aug 1;141(3):736–740. [PubMed] [Google Scholar]
- Jiang H., Takahara S., Takano Y., Machida M., Iwasaki A., Kokado Y., Kameoka H., Moutabarrik A., Ishibashi M., Sonoda T. In vitro immunosuppressive effect of deoxymethylspergualin. Transplant Proc. 1990 Aug;22(4):1633–1637. [PubMed] [Google Scholar]
- Kisielow P., Leiserson W., Von Boehmer H. Differentiation of thymocytes in fetal organ culture: analysis of phenotypic changes accompanying the appearance of cytolytic and interleukin 2-producing cells. J Immunol. 1984 Sep;133(3):1117–1123. [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Banan M., Harriss J. V., Hwang I., Woodward E., Youn H. J., Gottlieb P. D. Cis-acting DNA elements and cell type-specific nuclear proteins which may play a role in regulation of mouse CD8 alpha (Lyt-2) gene transcription. Int Immunol. 1994 Sep;6(9):1307–1321. doi: 10.1093/intimm/6.9.1307. [DOI] [PubMed] [Google Scholar]
- Lesley J., Trotter J., Hyman R. The Pgp-1 antigen is expressed on early fetal thymocytes. Immunogenetics. 1985;22(2):149–157. doi: 10.1007/BF00563512. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Migliorati G., Nicoletti I., Crocicchio F., Pagliacci C., D'Adamio F., Riccardi C. Heat shock induces apoptosis in mouse thymocytes and protects them from glucocorticoid-induced cell death. Cell Immunol. 1992 Sep;143(2):348–356. doi: 10.1016/0008-8749(92)90031-j. [DOI] [PubMed] [Google Scholar]
- Milarski K. L., Morimoto R. I. Expression of human HSP70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9517–9521. doi: 10.1073/pnas.83.24.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mivechi N. F., Trainor L. D., Hahn G. M. Purified mammalian HSP-70 KDA activates phosphoprotein phosphatases in vitro. Biochem Biophys Res Commun. 1993 Apr 30;192(2):954–963. doi: 10.1006/bbrc.1993.1508. [DOI] [PubMed] [Google Scholar]
- Morello D., Moore G., Salmon A. M., Yaniv M., Babinet C. Studies on the expression of an H-2K/human growth hormone fusion gene in giant transgenic mice. EMBO J. 1986 Aug;5(8):1877–1883. doi: 10.1002/j.1460-2075.1986.tb04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D. D., Duchaine J., Bourget L., Martin L. H. Changes in heat shock protein synthesis and heat sensitivity during mouse thymocyte development. Dev Genet. 1993;14(2):148–158. doi: 10.1002/dvg.1020140209. [DOI] [PubMed] [Google Scholar]
- Nossner E., Goldberg J. E., Naftzger C., Lyu S. C., Clayberger C., Krensky A. M. HLA-derived peptides which inhibit T cell function bind to members of the heat-shock protein 70 family. J Exp Med. 1996 Feb 1;183(2):339–348. doi: 10.1084/jem.183.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., Clayberger C., Zorn S. L., Ludwig D. S., Schoolnik G. K., Krensky A. M. Inhibition of alloreactive cytotoxic T lymphocytes by peptides from the alpha 2 domain of HLA-A2. Nature. 1987 Feb 12;325(6105):625–628. doi: 10.1038/325625a0. [DOI] [PubMed] [Google Scholar]
- Rüther U., Müller W., Sumida T., Tokuhisa T., Rajewsky K., Wagner E. F. c-fos expression interferes with thymus development in transgenic mice. Cell. 1988 Jun 17;53(6):847–856. doi: 10.1016/s0092-8674(88)90289-9. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Scollay R., Jacobs S., Jerabek L., Butcher E., Weissman I. T cell maturation: thymocyte and thymus migrant subpopulations defined with monoclonal antibodies to MHC region antigens. J Immunol. 1980 Jun;124(6):2845–2853. [PubMed] [Google Scholar]
- Scollay R., Shortman K. Thymocyte subpopulations: an experimental review, including flow cytometric cross-correlations between the major murine thymocyte markers. Thymus. 1983 Sep;5(5-6):245–295. [PubMed] [Google Scholar]
- Shortman K., Egerton M., Spangrude G. J., Scollay R. The generation and fate of thymocytes. Semin Immunol. 1990 Jan;2(1):3–12. [PubMed] [Google Scholar]
- Singh M. K., Yu J. Accumulation of a heat shock-like protein during differentiation of human erythroid cell line K562. Nature. 1984 Jun 14;309(5969):631–633. doi: 10.1038/309631a0. [DOI] [PubMed] [Google Scholar]
- Sumimoto R., Kamada N. Evidence that soluble class I antigen in donor serum induces the suppression of heart allograft rejection in rats. Immunol Lett. 1990 Oct;26(1):81–84. doi: 10.1016/0165-2478(90)90179-t. [DOI] [PubMed] [Google Scholar]
- Tepper M. A., Nadler S. G., Esselstyn J. M., Sterbenz K. G. Deoxyspergualin inhibits kappa light chain expression in 70Z/3 pre-B cells by blocking lipopolysaccharide-induced NF-kappa B activation. J Immunol. 1995 Sep 1;155(5):2427–2436. [PubMed] [Google Scholar]
- Zúiga-Pflücker J. C., Schwartz H. L., Lenardo M. J. Gene transcription in differentiating immature T cell receptor(neg) thymocytes resembles antigen-activated mature T cells. J Exp Med. 1993 Oct 1;178(4):1139–1149. doi: 10.1084/jem.178.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]






