Abstract
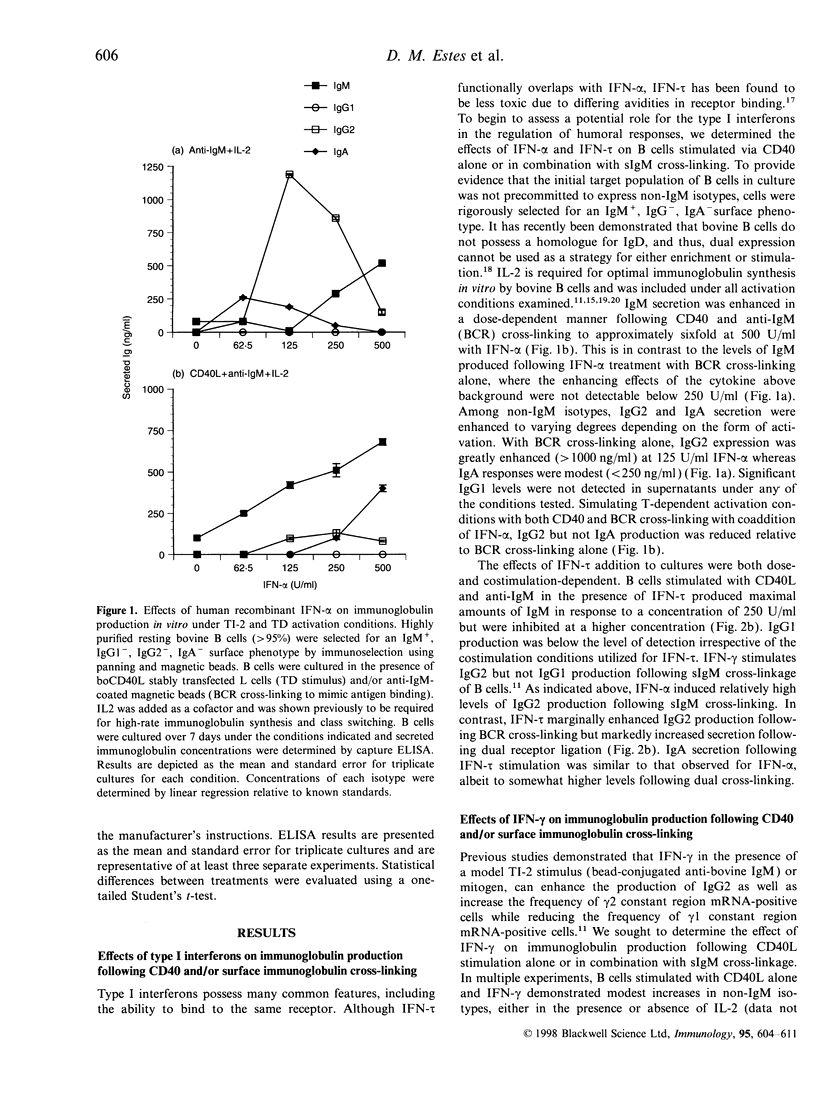
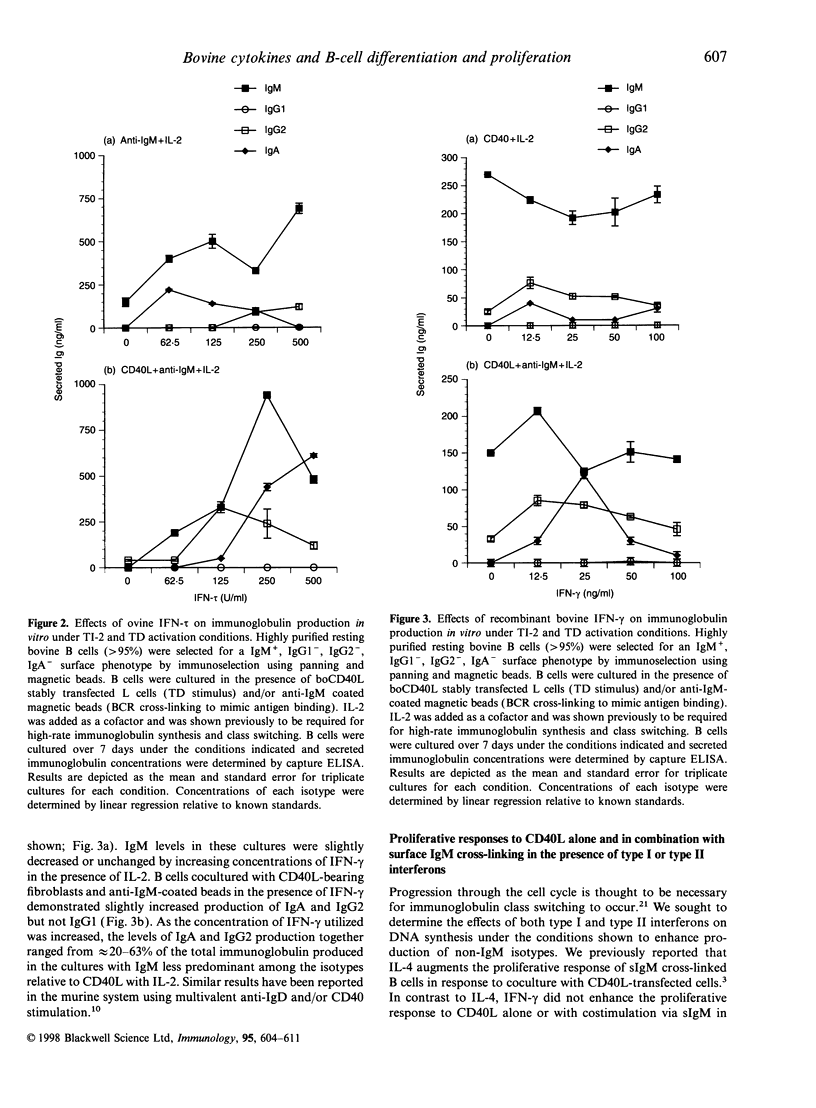
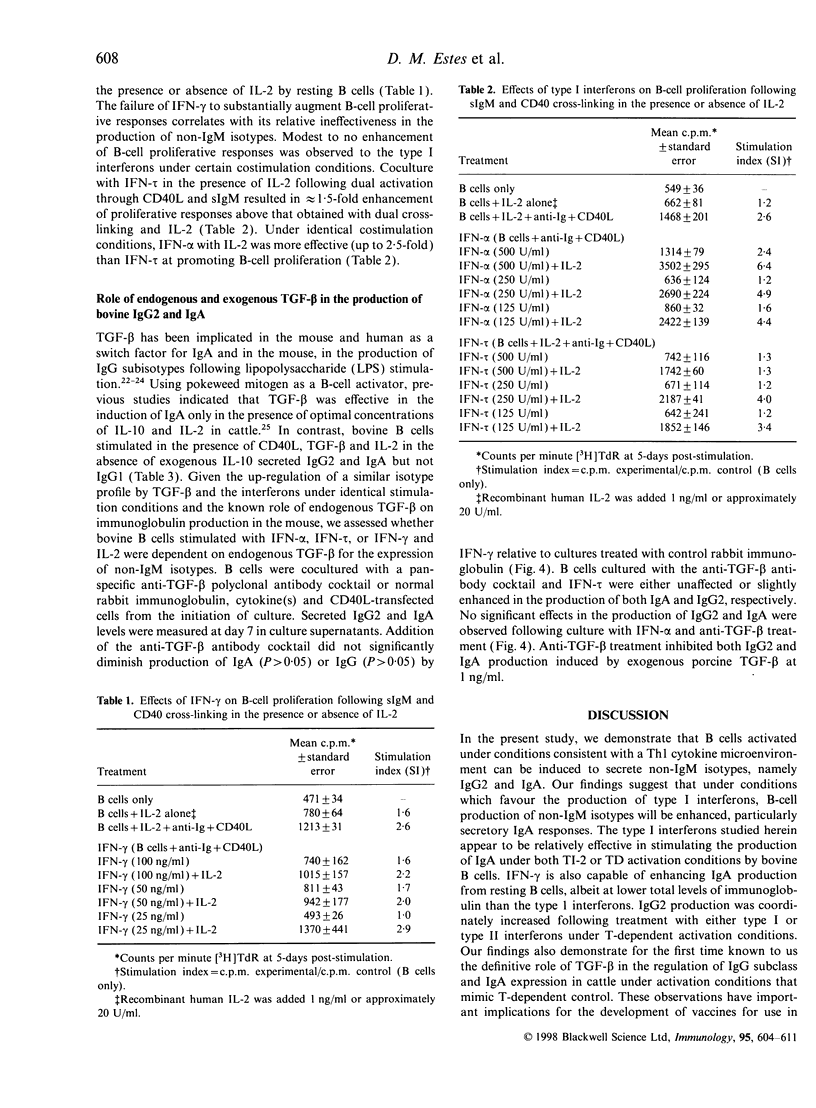
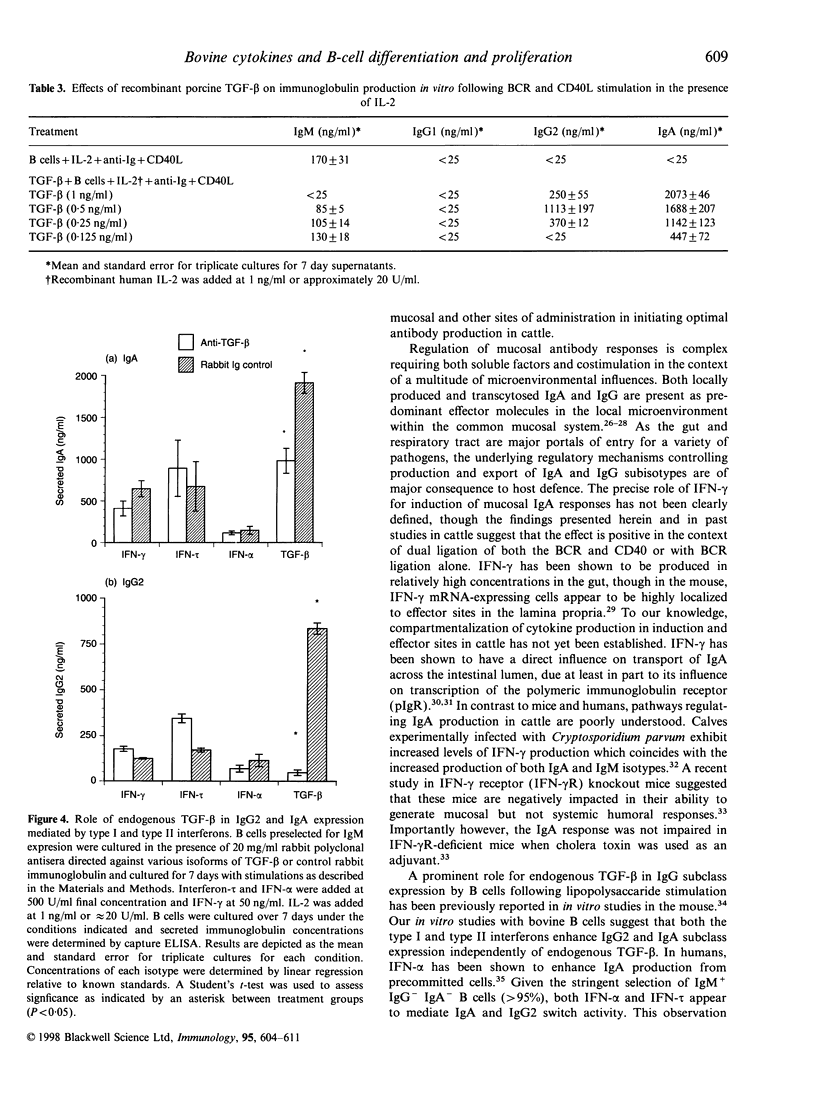
In this report, we sought to determine the role of selected type I interferons [interferon-alpha (IFN-alpha) and interferon-tau (IFN-tau)], IFN-gamma and transforming growth factor-beta (TGF-beta) in the regulation of bovine antibody responses. B cells were stimulated via CD40 in the presence or absence of B-cell receptor (BCR) cross-linking. IFN-alpha enhanced IgM, IgG2 and IgA responses but did not enhance IgG1 responses. BCR signalling alone was more effective at inducing IgG2 responses with IFN-alpha than dual cross-linking with CD40. Recombinant ovine IFN-tau was less effective at inducing IgG2 responses when compared with IFN-alpha, though IgA responses were similar in magnitude following BCR cross-linking. At higher concentrations, IFN-tau enhanced IgA responses greater than twofold over the levels observed with IFN-alpha. Previous studies have shown that addition of IFN-gamma to BCR or pokeweed mitogen-activated bovine B cells stimulates IgG2 production. However, following CD40 stimulation alone, IFN-gamma was relatively ineffective at stimulating high-rate synthesis of any non-IgM isotype. Dual cross-linking via CD40 and the BCR resulted in decreased synthesis of IgM with a concomitant increase in IgA and similar levels of IgG2 production to those obtained via the BCR alone. We also assessed the effects of endogenous and exogenous TGF-beta on immunoglobulin synthesis by bovine B cells. Exogenous TGF-beta stimulates both IgG2 and IgA production following CD40 and BCR cross-linking in the presence of IL-2. Blocking endogenous TGF-beta did not inhibit the up-regulation of IgG2 or IgA by interferons.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banchereau J., Bazan F., Blanchard D., Brière F., Galizzi J. P., van Kooten C., Liu Y. J., Rousset F., Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Bao S., Goldstone S., Husband A. J. Localization of IFN-gamma and IL-6 mRNA in murine intestine by in situ hybridization. Immunology. 1993 Dec;80(4):666–670. [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Lebman D. A., Shrader B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med. 1989 Sep 1;170(3):1039–1044. doi: 10.1084/jem.170.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins R. A., Oldham G. Recombinant human interleukin 2 induces proliferation and immunoglobulin secretion by bovine B-cells: tissue differences and preferential enhancement of immunoglobulin A. Vet Immunol Immunopathol. 1993 Feb;36(1):31–43. doi: 10.1016/0165-2427(93)90004-n. [DOI] [PubMed] [Google Scholar]
- Estes D. M., Closser N. M., Allen G. K. IFN-gamma stimulates IgG2 production from bovine B cells costimulated with anti-mu and mitogen. Cell Immunol. 1994 Apr 1;154(1):287–295. doi: 10.1006/cimm.1994.1078. [DOI] [PubMed] [Google Scholar]
- Estes D. M. Differentiation of B cells in the bovine. Role of cytokines in immunoglobulin isotype expression. Vet Immunol Immunopathol. 1996 Nov;54(1-4):61–67. doi: 10.1016/s0165-2427(96)05684-x. [DOI] [PubMed] [Google Scholar]
- Estes D. M., Hirano A., Heussler V. T., Dobbelaere D. A., Brown W. C. Expression and biological activities of bovine interleukin 4: effects of recombinant bovine interleukin 4 on T cell proliferation and B cell differentiation and proliferation in vitro. Cell Immunol. 1995 Jul;163(2):268–279. doi: 10.1006/cimm.1995.1126. [DOI] [PubMed] [Google Scholar]
- Estes D. M., Templeton J. W., Hunter D. M., Adams L. G. Production and use of murine monoclonal antibodies reactive with bovine IgM isotype and IgG subisotypes (IgG1, IgG2a and IgG2b) in assessing immunoglobulin levels in serum of cattle. Vet Immunol Immunopathol. 1990 May;25(1):61–72. doi: 10.1016/0165-2427(90)90110-e. [DOI] [PubMed] [Google Scholar]
- Flores-Romo L., Millsum M. J., Gillis S., Stubbs P., Sykes C., Gordon J. Immunoglobulin isotype production by cycling human B lymphocytes in response to recombinant cytokines and anti-IgM. Immunology. 1990 Mar;69(3):342–347. [PMC free article] [PubMed] [Google Scholar]
- Foote L. C., Howard R. G., Marshak-Rothstein A., Rothstein T. L. IL-4 induces Fas resistance in B cells. J Immunol. 1996 Oct 1;157(7):2749–2753. [PubMed] [Google Scholar]
- Hirano A., Brown W. C., Estes D. M. Cloning, expression and biological function of the bovine CD40 homologue: role in B-lymphocyte growth and differentiation in cattle. Immunology. 1997 Feb;90(2):294–300. doi: 10.1046/j.1365-2567.1997.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A., Brown W. C., Trigona W., Tuo W., Estes D. M. Kinetics of expression and subset distribution of the TNF superfamily members CD40 ligand and Fas ligand on T lymphocytes in cattle. Vet Immunol Immunopathol. 1998 Feb 27;61(2-4):251–263. doi: 10.1016/s0165-2427(97)00155-4. [DOI] [PubMed] [Google Scholar]
- Ju S. T., Cui H., Panka D. J., Ettinger R., Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4185–4189. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer D. R., Sutherland R. M., Bao S., Husband A. J. Cytokine mediated effects in mucosal immunity. Immunol Cell Biol. 1995 Oct;73(5):389–396. doi: 10.1038/icb.1995.61. [DOI] [PubMed] [Google Scholar]
- Lundgren M., Ström L., Bergquist L. O., Skog S., Heiden T., Stavnezer J., Severinson E. Cell cycle regulation of immunoglobulin class switch recombination and germ-line transcription: potential role of Ets family members. Eur J Immunol. 1995 Jul;25(7):2042–2051. doi: 10.1002/eji.1830250736. [DOI] [PubMed] [Google Scholar]
- McIntyre T. M., Klinman D. R., Rothman P., Lugo M., Dasch J. R., Mond J. J., Snapper C. M. Transforming growth factor beta 1 selectivity stimulates immunoglobulin G2b secretion by lipopolysaccharide-activated murine B cells. J Exp Med. 1993 Apr 1;177(4):1031–1037. doi: 10.1084/jem.177.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., Russell M. W. Mucosal immunoglobulins and their contribution to defence mechanisms: an overview. Biochem Soc Trans. 1997 May;25(2):457–462. doi: 10.1042/bst0250457. [DOI] [PubMed] [Google Scholar]
- Naessens J. Surface Ig on B lymphocytes from cattle and sheep. Int Immunol. 1997 Mar;9(3):349–354. doi: 10.1093/intimm/9.3.349. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Roy M., Shepherd D. M., Stamenkovic I., Ledbetter J. A., Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noelle R. J. The role of gp39 (CD40L) in immunity. Clin Immunol Immunopathol. 1995 Sep;76(3 Pt 2):S203–S207. doi: 10.1016/s0090-1229(95)90234-1. [DOI] [PubMed] [Google Scholar]
- Piskurich J. F., France J. A., Tamer C. M., Willmer C. A., Kaetzel C. S., Kaetzel D. M. Interferon-gamma induces polymeric immunoglobulin receptor mRNA in human intestinal epithelial cells by a protein synthesis dependent mechanism. Mol Immunol. 1993 Mar;30(4):413–421. doi: 10.1016/0161-5890(93)90071-i. [DOI] [PubMed] [Google Scholar]
- Piskurich J. F., Youngman K. R., Phillips K. M., Hempen P. M., Blanchard M. H., France J. A., Kaetzel C. S. Transcriptional regulation of the human polymeric immunoglobulin receptor gene by interferon-gamma. Mol Immunol. 1997 Jan;34(1):75–91. doi: 10.1016/s0161-5890(96)00079-x. [DOI] [PubMed] [Google Scholar]
- Pontzer C. H., Torres B. A., Vallet J. L., Bazer F. W., Johnson H. M. Antiviral activity of the pregnancy recognition hormone ovine trophoblast protein-1. Biochem Biophys Res Commun. 1988 Apr 29;152(2):801–807. doi: 10.1016/s0006-291x(88)80109-8. [DOI] [PubMed] [Google Scholar]
- Purkerson J., Isakson P. A two-signal model for regulation of immunoglobulin isotype switching. FASEB J. 1992 Nov;6(14):3245–3252. doi: 10.1096/fasebj.6.14.1385241. [DOI] [PubMed] [Google Scholar]
- Rogge L., Barberis-Maino L., Biffi M., Passini N., Presky D. H., Gubler U., Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997 Mar 3;185(5):825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein T. L., Wang J. K., Panka D. J., Foote L. C., Wang Z., Stanger B., Cui H., Ju S. T., Marshak-Rothstein A. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature. 1995 Mar 9;374(6518):163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Rosas F., Moorman M. A., Jin L., Shanebeck K., Klinman D. M., Kehry M. R., Mond J. J., Maliszewski C. R. IFN-gamma is a potent inducer of Ig secretion by sort-purified murine B cells activated through the mIg, but not the CD40, signaling pathway. Int Immunol. 1996 Jun;8(6):877–885. doi: 10.1093/intimm/8.6.877. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Waegell W., Beernink H., Dasch J. R. Transforming growth factor-beta 1 is required for secretion of IgG of all subclasses by LPS-activated murine B cells in vitro. J Immunol. 1993 Nov 1;151(9):4625–4636. [PubMed] [Google Scholar]
- Sonoda E., Matsumoto R., Hitoshi Y., Ishii T., Sugimoto M., Araki S., Tominaga A., Yamaguchi N., Takatsu K. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med. 1989 Oct 1;170(4):1415–1420. doi: 10.1084/jem.170.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam P. S., Khan S. A., Pontzer C. H., Johnson H. M. Differential recognition of the type I interferon receptor by interferons tau and alpha is responsible for their disparate cytotoxicities. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12270–12274. doi: 10.1073/pnas.92.26.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Vancott J. L., Okahashi N., Marinaro M., Kiyono H., Fujihashi K., Jackson R. J., Chatfield S. N., Bluethmann H., McGhee J. R. The role of Th1 and Th2 cells for mucosal IgA responses. Ann N Y Acad Sci. 1996 Feb 13;778:64–71. doi: 10.1111/j.1749-6632.1996.tb21115.x. [DOI] [PubMed] [Google Scholar]
- de Graaf D. C., Peeters J. E. Specific interferon-gamma, IgA and IgM responses after experimental infection of neonatal calves with Cryptosporidium parvum. Int J Parasitol. 1997 Jan;27(1):131–134. doi: 10.1016/s0020-7519(96)00167-1. [DOI] [PubMed] [Google Scholar]
- van Vlasselaer P., Punnonen J., de Vries J. E. Transforming growth factor-beta directs IgA switching in human B cells. J Immunol. 1992 Apr 1;148(7):2062–2067. [PubMed] [Google Scholar]



