Abstract
Our previous study has shown that the rapid and sufficient activation of complement by Salmonella lipopolysaccharide occurs in genetically resistant (Ityr) A/J mice. To assess whether the level of complement activation by a virulent strain of Salmonella typhimurium regulates the level of murine natural resistance, we compared levels of serum complement activation by S. typhimurium and kinetics of serum-opsonized S. typhimurium grown in macrophages using several strains of resistant (Ityr) and susceptible (Itys) mice. Itys macrophages killed intracellular S. typhimurium to the same extent as did Ityr macrophages when the pathogen was opsonized with Ityr serum. Opsonization of S. typhimurium with Itys serum reduced intracellular killing activity in Ityr macrophages to the same level as seen with Itys macrophages. Incubation of S. typhimurium with 25% Mg2+ EGTA (5 mm MgCl2-3 mm ethylene glycol-bis (beta-aminotheyl either)-N,N,N',N'-tetraacetic acid)-chelated Ityr serum resulted in higher levels of C3 deposition onto the surface of this bacteria, C3b generation and also C3 consumption, compared with that with Mg2+ EGTA-chelated Itys serum. Opsonization of S. typhimurium with A/J serum prior to infection increased early resistance in Itys mice. Infection with a virulent strain of S. typhimurium induced the expression of interleukin-10 (IL-10) mRNA at higher levels in C57BL/6 mice than in A/J mice. However, opsonization of S. typhimurium with A/J serum decreased bacterial growth in the spleen of C57BL/6 mice to the same level as observed for A/J mice in association with decreased expression levels of IL-10 mRNA. Moreover, administration of anti-C3 antibodies reduced the resistance of A/J mice in association with a decrease in serum levels of C3. These results indicate that the high level of complement activation via the alternative pathway in Ityr serum by a virulent strain of S. typhimurium reduces the virulence of this pathogen, which may contribute to the full expression of Ity phenotype in Ityr mice.
Full text
PDF


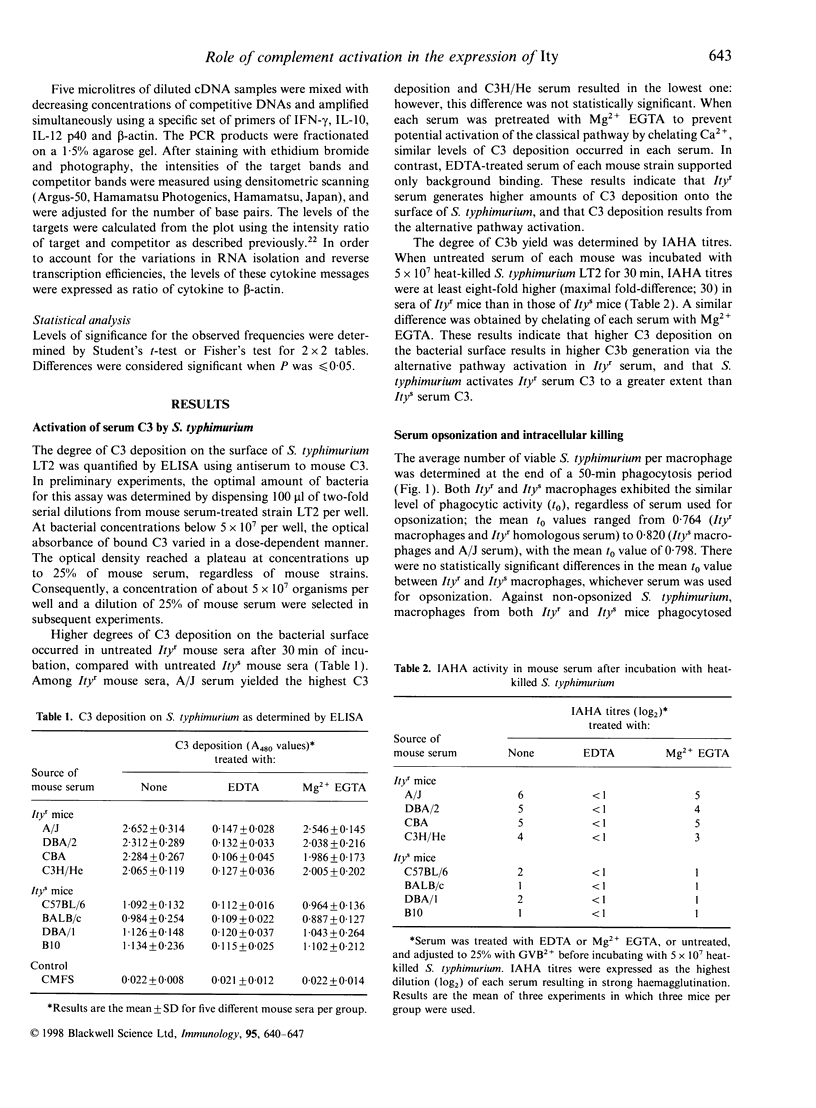
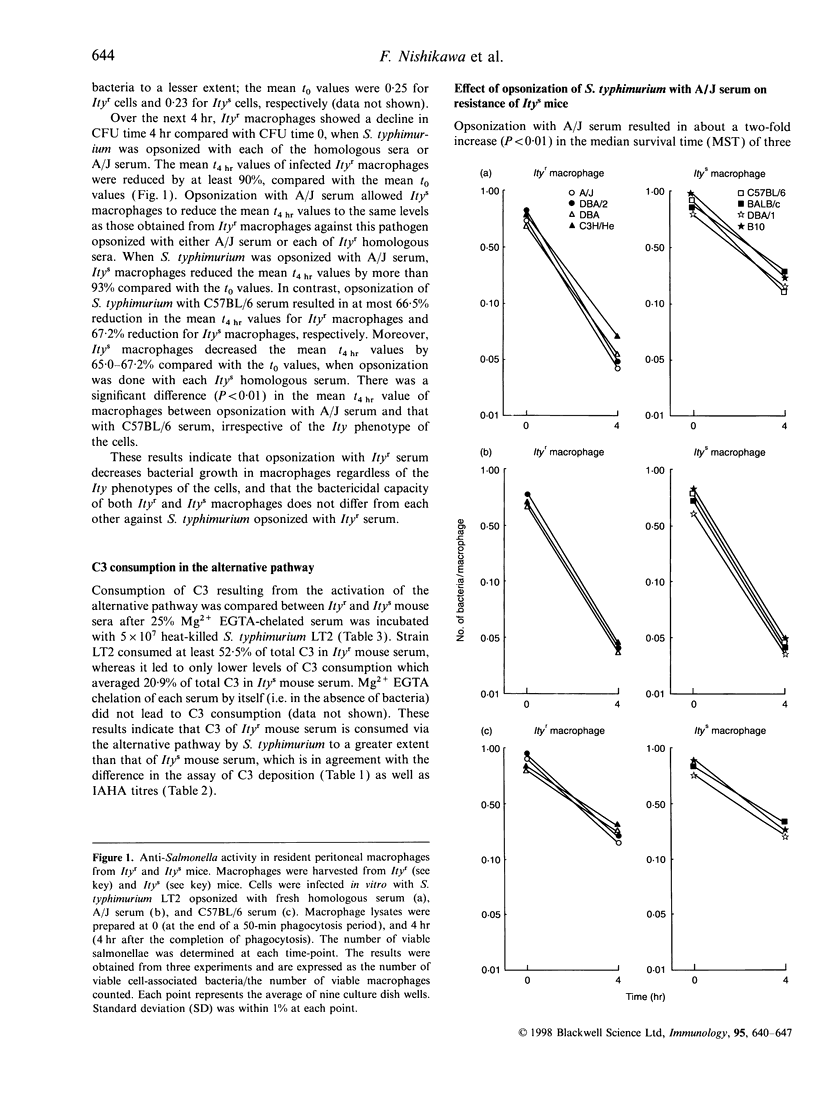

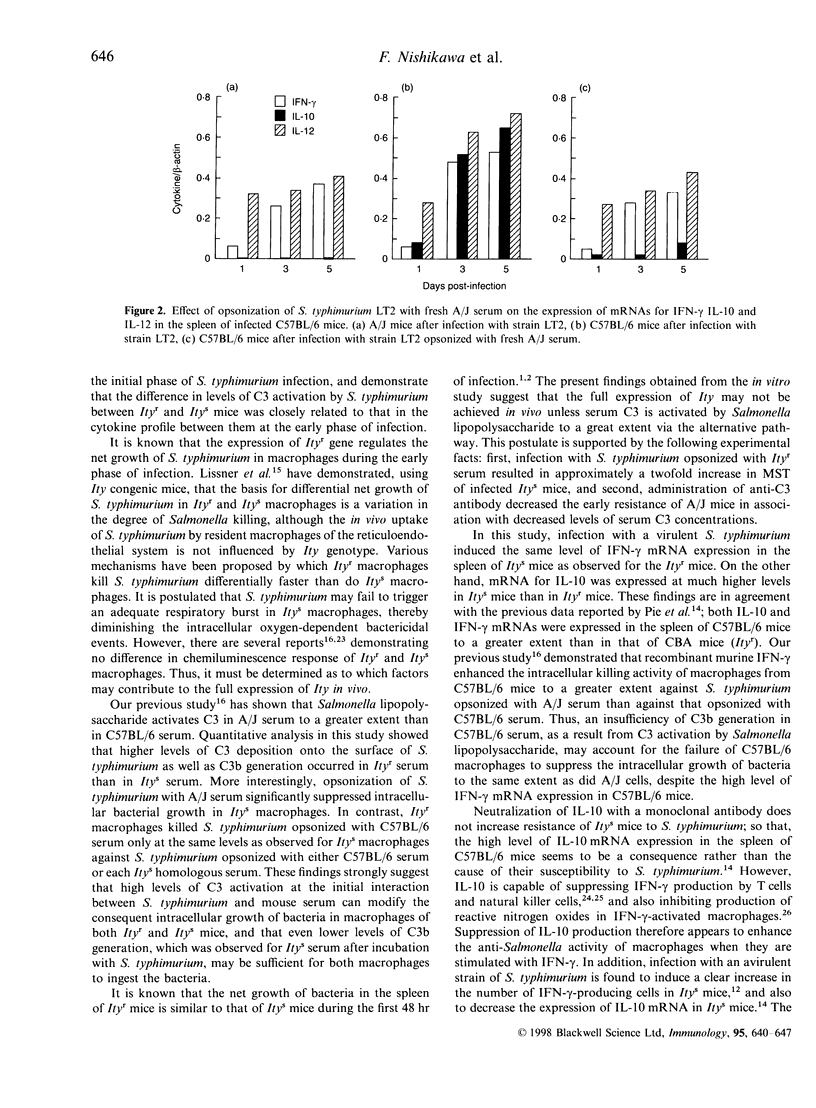

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu J. S., Kanangat S., Rouse B. T. Limitations and modifications of quantitative polymerase chain reaction. Application to measurement of multiple mRNAs present in small amounts of sample RNA. J Immunol Methods. 1993 Oct 15;165(2):207–216. doi: 10.1016/0022-1759(93)90346-9. [DOI] [PubMed] [Google Scholar]
- Barrera L. F., Kramnik I., Skamene E., Radzioch D. Nitrite production by macrophages derived from BCG-resistant and -susceptible congenic mouse strains in response to IFN-gamma and infection with BCG. Immunology. 1994 Jul;82(3):457–464. [PMC free article] [PubMed] [Google Scholar]
- Barton C. H., White J. K., Roach T. I., Blackwell J. M. NH2-terminal sequence of macrophage-expressed natural resistance-associated macrophage protein (Nramp) encodes a proline/serine-rich putative Src homology 3-binding domain. J Exp Med. 1994 May 1;179(5):1683–1687. doi: 10.1084/jem.179.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbernou N., Nauciel C. Influence of mouse genotype and bacterial virulence in the generation of interferon-gamma-producing cells during the early phase of Salmonella typhimurium infection. Immunology. 1994 Oct;83(2):245–249. [PMC free article] [PubMed] [Google Scholar]
- Benjamin W. H., Jr, Hall P., Roberts S. J., Briles D. E. The primary effect of the Ity locus is on the rate of growth of Salmonella typhimurium that are relatively protected from killing. J Immunol. 1990 Apr 15;144(8):3143–3151. [PubMed] [Google Scholar]
- Blumenstock E., Jann K. Natural resistance of mice to Salmonella typhimurium: bactericidal activity and chemiluminescence response of murine peritoneal macrophages. J Gen Microbiol. 1981 Jul;125(1):173–183. doi: 10.1099/00221287-125-1-173. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Vodovotz Y., Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991 Dec 1;174(6):1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991 Jan;132(1):150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- Hamada K., Kita E., Sawaki M., Mikasa K., Narita N. Antitumor effect of erythromycin in mice. Chemotherapy. 1995 Jan-Feb;41(1):59–69. doi: 10.1159/000239325. [DOI] [PubMed] [Google Scholar]
- Hormaeche C. E. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979 Jun;37(2):311–318. [PMC free article] [PubMed] [Google Scholar]
- Hsu D. H., Moore K. W., Spits H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN-gamma synthesis and lymphokine-activated killer activity. Int Immunol. 1992 May;4(5):563–569. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- Kanangat S., Solomon A., Rouse B. T. Use of quantitative polymerase chain reaction to quantitate cytokine messenger RNA molecules. Mol Immunol. 1992 Oct;29(10):1229–1236. doi: 10.1016/0161-5890(92)90059-7. [DOI] [PubMed] [Google Scholar]
- Kita E., Emoto M., Oku D., Nishikawa F., Hamuro A., Kamikaidou N., Kashiba S. Contribution of interferon gamma and membrane-associated interleukin 1 to the resistance to murine typhoid of Ityr mice. J Leukoc Biol. 1992 Mar;51(3):244–250. doi: 10.1002/jlb.51.3.244. [DOI] [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Saxén H., Mäkelä P. H., Leive L. Complement activation by polysaccharide of lipopolysaccharide: an important virulence determinant of salmonellae. Infect Immun. 1983 Aug;41(2):563–569. doi: 10.1128/iai.41.2.563-569.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner C. R., Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983 Dec;131(6):3006–3013. [PubMed] [Google Scholar]
- Mosmann T. R. Regulation of immune responses by T cells with different cytokine secretion phenotypes: role of a new cytokine, cytokine synthesis inhibitory factor (IL10). Int Arch Allergy Appl Immunol. 1991;94(1-4):110–115. doi: 10.1159/000235340. [DOI] [PubMed] [Google Scholar]
- Muotiala A., Mäkelä P. H. The role of IFN-gamma in murine Salmonella typhimurium infection. Microb Pathog. 1990 Feb;8(2):135–141. doi: 10.1016/0882-4010(90)90077-4. [DOI] [PubMed] [Google Scholar]
- Nakano A., Kita E., Kashiba S. Different sensitivity of complement to Salmonella typhimurium accounts for the difference in natural resistance to murine typhoid between A/J and C57BL/6 mice. Microbiol Immunol. 1995;39(2):95–103. doi: 10.1111/j.1348-0421.1995.tb02175.x. [DOI] [PubMed] [Google Scholar]
- Nauciel C., Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992 Feb;60(2):450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pie S., Matsiota-Bernard P., Truffa-Bachi P., Nauciel C. Gamma interferon and interleukin-10 gene expression in innately susceptible and resistant mice during the early phase of Salmonella typhimurium infection. Infect Immun. 1996 Mar;64(3):849–854. doi: 10.1128/iai.64.3.849-854.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Locating salmonella resistance gene on mouse chromosome 1. Clin Exp Immunol. 1979 Jul;37(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Unkles S. E., Hawker K. L., Grieve C., Campbell E. I., Montague P., Kinghorn J. R. crnA encodes a nitrate transporter in Aspergillus nidulans. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):204–208. doi: 10.1073/pnas.88.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S. M., Malo D., Vogan K., Skamene E., Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993 May 7;73(3):469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Yamai S., Obara Y., Nikkawa T., Shimoda Y., Miyamoto Y. Preservation of Neisseria gonorrhoeae by the gelatin-disc method. Br J Vener Dis. 1979 Apr;55(2):90–93. doi: 10.1136/sti.55.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]


