Abstract
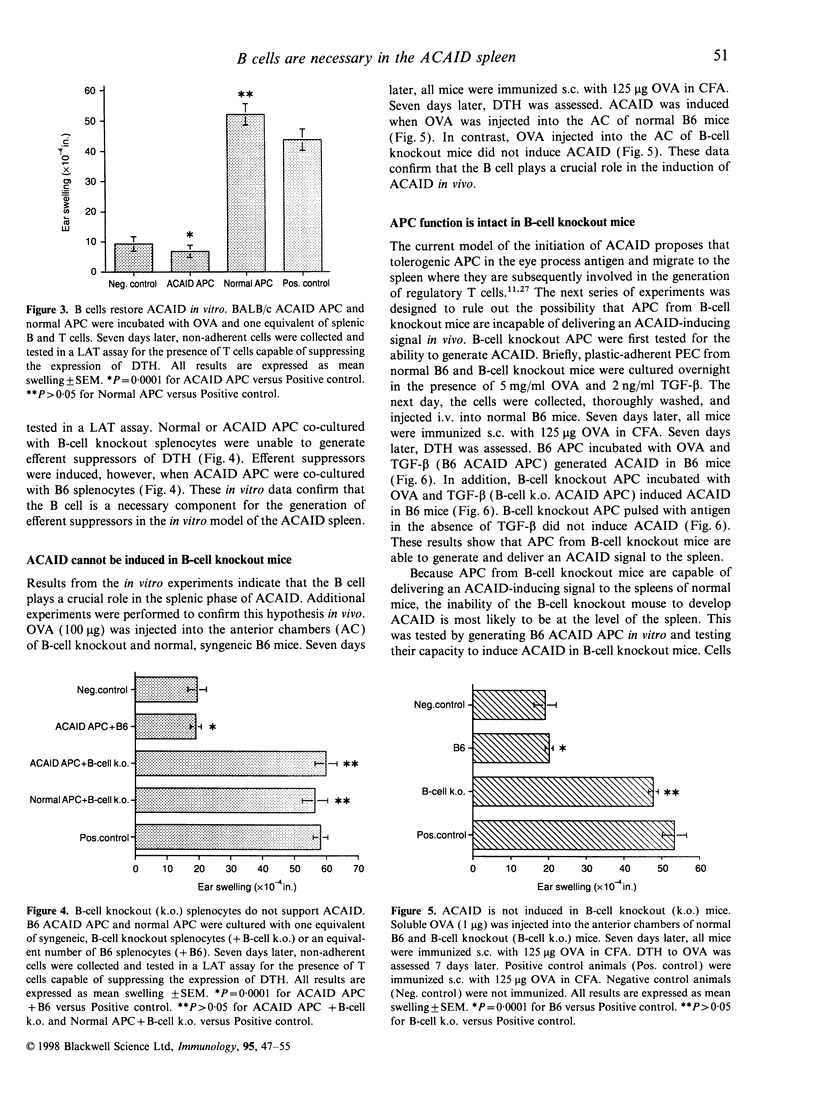
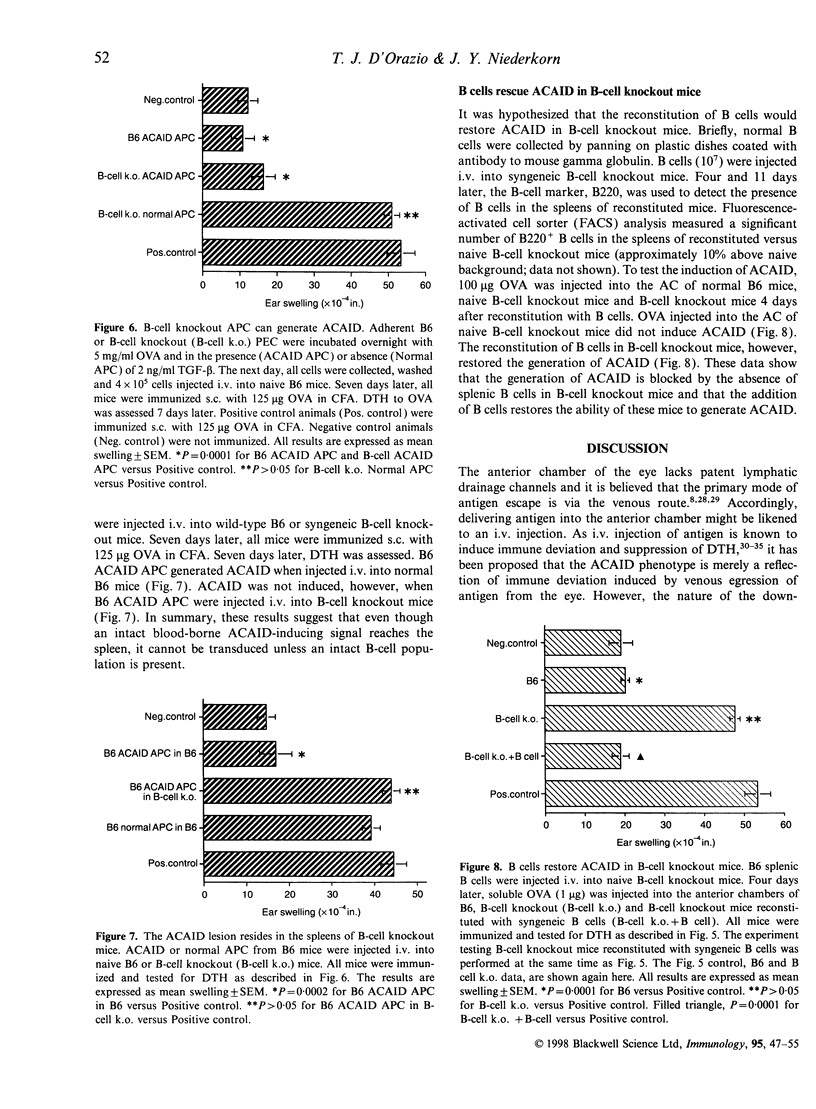
Ocular immune privilege is the result of a number of protective mechanisms, including a specialized immune response to antigen encountered in the anterior chamber of the eye. Anterior chamber-associated immune deviation, or ACAID, is characterized by the antigen-specific, selective down-regulation of systemic cell-mediated and humoral immune responses. One current hypothesis of the initiation of ACAID predicts that ocular APC process antigen and then migrate out of the eye and to the spleen where various regulatory T-cell populations are generated. A novel in vitro model of the ACAID spleen was developed to study the cells involved in the generation of suppressed T-cell immunity. ACAID APC co-cultured with whole splenocytes or splenic B and T cells induced efferent suppressors of delayed-type hypersensitivity (DTH). However, ACAID APC co-cultured with splenic T cells did not generate efferent suppressors of DTH. The requirement for B cells was confirmed with B-cell knockout mice. ACAID APC co-cultured with splenocytes from B-cell knockout mice did not induce efferent suppressors of DTH. Moreover, ACAID could not be induced in B-cell knockout mice in vivo. The reconstitution of B-cell knockout mice with wild-type B cells restored ACAID. In summary, these data confirm the role for B cells in the splenic phase of ACAID. A putative mechanism predicts that ACAID APC release antigenic peptides to B cells in the spleen. B cells then present antigen in a tolerogenic manner leading to the generation of regulatory T cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B., Germain R. N. A single major pathway of T-lymphocyte interactions in antigen-specific immune suppression. Scand J Immunol. 1981;13(1):1–10. doi: 10.1111/j.1365-3083.1981.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Eynon E. E., Parker D. C. Do small B cells induce tolerance? Transplant Proc. 1991 Feb;23(1 Pt 1):729–730. [PubMed] [Google Scholar]
- Eynon E. E., Parker D. C. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992 Jan 1;175(1):131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson T. A., Herndon J. M. The immune response and the eye: the ACAID inducing signal is dependent on the nature of the antigen. Invest Ophthalmol Vis Sci. 1994 Jun;35(7):3085–3093. [PubMed] [Google Scholar]
- Gajewski T. F., Pinnas M., Wong T., Fitch F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991 Mar 15;146(6):1750–1758. [PubMed] [Google Scholar]
- Gieni R. S., Umetsu D. T., DeKruyff R. H. Ly1- (CD5-) B cells produce interleukin (IL)-10. Cell Immunol. 1997 Feb 1;175(2):164–170. doi: 10.1006/cimm.1996.1060. [DOI] [PubMed] [Google Scholar]
- Griffith T. S., Brunner T., Fletcher S. M., Green D. R., Ferguson T. A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995 Nov 17;270(5239):1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- Hara Y., Caspi R. R., Wiggert B., Dorf M., Streilein J. W. Analysis of an in vitro-generated signal that induces systemic immune deviation similar to that elicited by antigen injected into the anterior chamber of the eye. J Immunol. 1992 Sep 1;149(5):1531–1538. [PubMed] [Google Scholar]
- Hara Y., Okamoto S., Rouse B., Streilein J. W. Evidence that peritoneal exudate cells cultured with eye-derived fluids are the proximate antigen-presenting cells in immune deviation of the ocular type. J Immunol. 1993 Nov 15;151(10):5162–5171. [PubMed] [Google Scholar]
- Kaplan H. J., Streilein J. W. Do immunologically privileged sites require a functioning spleen? Nature. 1974 Oct 11;251(5475):553–554. doi: 10.1038/251553a0. [DOI] [PubMed] [Google Scholar]
- Kaplan H. J., Streilein J. W., Stevens T. R. Transplantation immunology of the anterior chamber of the eye. II. Immune response to allogeneic cells. J Immunol. 1975 Sep;115(3):805–810. [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Kosiewicz M. M., Okamoto S., Miki S., Ksander B. R., Shimizu T., Streilein J. W. Imposing deviant immunity on the presensitized state. J Immunol. 1994 Oct 1;153(7):2962–2973. [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Influence of dose and route of antigen injection on the immunological induction of T cells. J Exp Med. 1974 Mar 1;139(3):528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Potentiation of T-cell-mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974 Jun 1;139(6):1529–1539. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay A. E., DeKruyff R. H., Goodnow C. C., Umetsu D. T. Antigen-specific B cells preferentially induce CD4+ T cells to produce IL-4. J Immunol. 1997 May 1;158(9):4171–4179. [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Miller T. E., Ishibashi T. Feedback inhibition of specifically sensitized lymphocytes. J Exp Med. 1974 Mar 1;139(3):543–559. doi: 10.1084/jem.139.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn J. Y. Immune privilege and immune regulation in the eye. Adv Immunol. 1990;48:191–226. doi: 10.1016/s0065-2776(08)60755-5. [DOI] [PubMed] [Google Scholar]
- Niederkorn J. Y., Mayhew E. Role of splenic B cells in the immune privilege of the anterior chamber of the eye. Eur J Immunol. 1995 Oct;25(10):2783–2787. doi: 10.1002/eji.1830251011. [DOI] [PubMed] [Google Scholar]
- Niederkorn J. Y., Streilein J. W. Induction of anterior chamber-associated immune deviation (ACAID) by allogeneic intraocular tumors does not require splenic metastases. J Immunol. 1982 Jun;128(6):2470–2474. [PubMed] [Google Scholar]
- Noble A., Zhao Z. S., Cantor H. Suppression of immune responses by CD8 cells. II. Qa-1 on activated B cells stimulates CD8 cell suppression of T helper 2 responses. J Immunol. 1998 Jan 15;160(2):566–571. [PubMed] [Google Scholar]
- O'Garra A., Howard M. IL-10 production by CD5 B cells. Ann N Y Acad Sci. 1992 May 4;651:182–199. doi: 10.1111/j.1749-6632.1992.tb24615.x. [DOI] [PubMed] [Google Scholar]
- Parker D. C., Eynon E. E. Antigen presentation in acquired immunological tolerance. FASEB J. 1991 Oct;5(13):2777–2784. doi: 10.1096/fasebj.5.13.1916102. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Jensen A. D., Silverstein A. M. Antibody formation by single cells during experimental immunogenic uveitis. Invest Ophthalmol. 1969 Aug;8(4):373–380. [PubMed] [Google Scholar]
- Streilein J. W. Immune privilege as the result of local tissue barriers and immunosuppressive microenvironments. Curr Opin Immunol. 1993 Jun;5(3):428–432. doi: 10.1016/0952-7915(93)90064-y. [DOI] [PubMed] [Google Scholar]
- Streilein J. W. Immunological non-responsiveness and acquisition of tolerance in relation to immune privilege in the eye. Eye (Lond) 1995;9(Pt 2):236–240. doi: 10.1038/eye.1995.46. [DOI] [PubMed] [Google Scholar]
- Streilein J. W., Ksander B. R., Taylor A. W. Immune deviation in relation to ocular immune privilege. J Immunol. 1997 Apr 15;158(8):3557–3560. [PubMed] [Google Scholar]
- Streilein J. W., Niederkorn J. Y. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med. 1981 May 1;153(5):1058–1067. doi: 10.1084/jem.153.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein J. W., Okamoto S., Hara Y., Kosiewicz M., Ksander B. Blood-borne signals that induce anterior chamber-associated immune deviation after intracameral injection of antigen. Invest Ophthalmol Vis Sci. 1997 Oct;38(11):2245–2254. [PubMed] [Google Scholar]
- Streilein J. W., Streilein J. S. Immunologic reactivity to weak transplantation antigens: factors which favor sensitization rather than tolerance. J Natl Cancer Inst. 1973 Nov;51(5):1589–1595. doi: 10.1093/jnci/51.5.1589. [DOI] [PubMed] [Google Scholar]
- Sy M. S., Miller S. D., Kowach H. B., Claman H. N. A splenic requirement for the generation of suppressor T cells. J Immunol. 1977 Dec;119(6):2095–2099. [PubMed] [Google Scholar]
- Takeuchi M., Kosiewicz M. M., Alard P., Streilein J. W. On the mechanisms by which transforming growth factor-beta 2 alters antigen-presenting abilities of macrophages on T cell activation. Eur J Immunol. 1997 Jul;27(7):1648–1656. doi: 10.1002/eji.1830270709. [DOI] [PubMed] [Google Scholar]
- Wilbanks G. A., Mammolenti M., Streilein J. W. Studies on the induction of anterior chamber-associated immune deviation (ACAID). II. Eye-derived cells participate in generating blood-borne signals that induce ACAID. J Immunol. 1991 May 1;146(9):3018–3024. [PubMed] [Google Scholar]
- Wilbanks G. A., Mammolenti M., Streilein J. W. Studies on the induction of anterior chamber-associated immune deviation (ACAID). III. Induction of ACAID depends upon intraocular transforming growth factor-beta. Eur J Immunol. 1992 Jan;22(1):165–173. doi: 10.1002/eji.1830220125. [DOI] [PubMed] [Google Scholar]
- Wilbanks G. A., Streilein J. W. Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID) induced by soluble antigen. Evidence of two functionally and phenotypically distinct T-suppressor cell populations. Immunology. 1990 Nov;71(3):383–389. [PMC free article] [PubMed] [Google Scholar]
- Wilbanks G. A., Streilein J. W. Fluids from immune privileged sites endow macrophages with the capacity to induce antigen-specific immune deviation via a mechanism involving transforming growth factor-beta. Eur J Immunol. 1992 Apr;22(4):1031–1036. doi: 10.1002/eji.1830220423. [DOI] [PubMed] [Google Scholar]
- Wilbanks G. A., Streilein J. W. Macrophages capable of inducing anterior chamber associated immune deviation demonstrate spleen-seeking migratory properties. Reg Immunol. 1992 May-Jun;4(3):130–137. [PubMed] [Google Scholar]
- Wilbanks G. A., Streilein J. W. Studies on the induction of anterior chamber-associated immune deviation (ACAID). 1. Evidence that an antigen-specific, ACAID-inducing, cell-associated signal exists in the peripheral blood. J Immunol. 1991 Apr 15;146(8):2610–2617. [PubMed] [Google Scholar]
- Wilbanks G. A., Streilein J. W. The differing patterns of antigen release and local retention following anterior chamber and intravenous inoculation of soluble antigen. Evidence that the eye acts as an antigen depot. Reg Immunol. 1989 Nov-Dec;2(6):390–398. [PubMed] [Google Scholar]
- Yao Y. F., Inoue Y., Miyazaki D., Hara Y., Shimomura Y., Tano Y., Ohashi Y. The antigen-bearing eye and the spleen are indispensable in maintaining anterior chamber-associated immune deviation. Invest Ophthalmol Vis Sci. 1997 Feb;38(2):534–539. [PubMed] [Google Scholar]



