Abstract
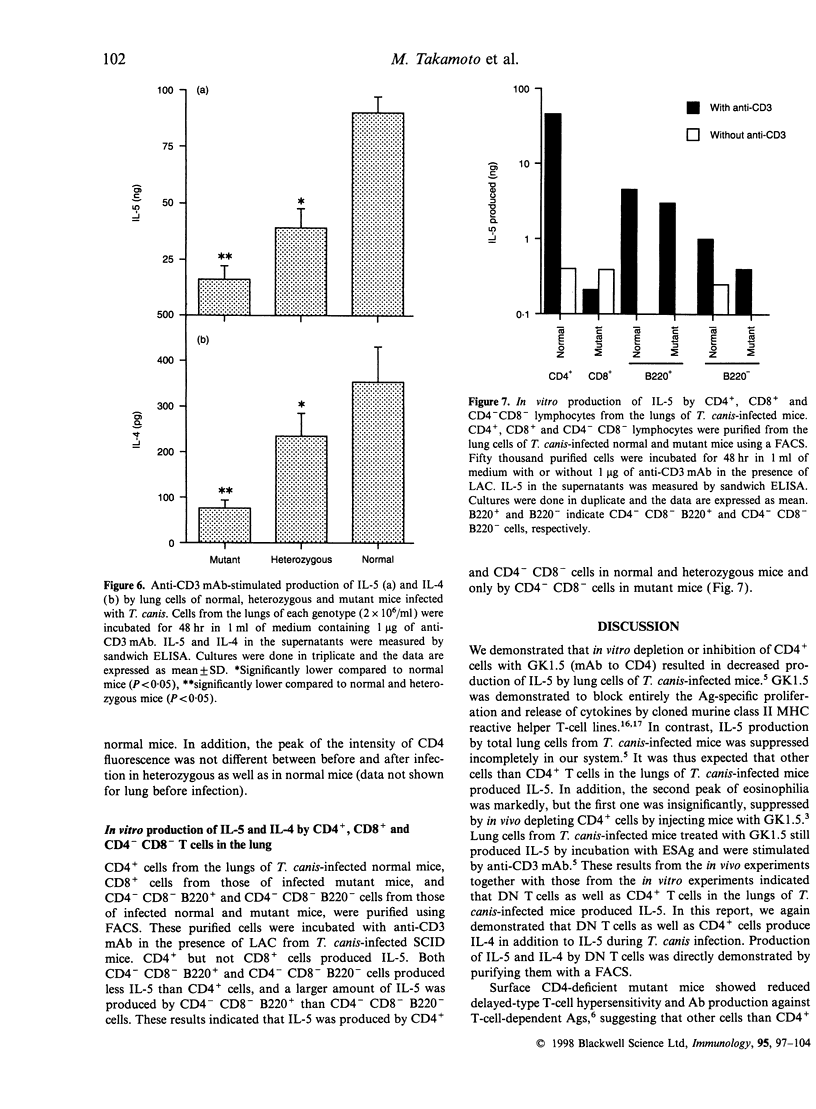
Mutant mice deficient in CD4+ T cells and their normal and heterozygous littermates were infected with Toxocara canis, and compared for eosinophilia, total and Toxocara-specific immunoglobulin E (IgE) production, and in vitro cytokine production by lung cells. The numbers of eosinophils in the peripheral blood of normal and heterozygous mice peaked on days 10 and 21, although mutant mice showed eosinophilia with a peak on day 10. This indicates that the first peak on day 10 is CD4 independent and the second peak is CD4 dependent. Before infection, the levels of total IgE had no significant difference among the three groups of mice. Total and Toxocara-specific IgE in all genotypes of mice increased after infection, and was the highest in normal mice and the lowest in mutant mice. In vitro production of interleukin (IL)-5 and IL-4 by total lung cells was the highest in normal mice and the lowest in mutant mice. CD4+ and CD4- CD8- T lymphocytes, but not CD8+ T lymphocytes produced IL-5 and IL-4 when incubated with anti-CD3 monoclonal antibody (mAb) and lung-adherent cells. These results indicated that IL-5 and IL-4 were produced mainly by CD4+ cells and partly by CD4- CD8- cells, but not by CD8+ cells. In addition, cytokine production by CD4+ cells was affected by the number of CD4 molecules on their surface.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Díez-Orejas R., Ballester S., Feito M. J., Ojeda G., Criado G., Ronda M., Portolés P., Rojo J. M. Genetic and immunochemical evidence for CD4-dependent association of p56lck with the alpha beta T-cell receptor (TCR): regulation of TCR-induced activation. EMBO J. 1994 Jan 1;13(1):90–99. doi: 10.1002/j.1460-2075.1994.tb06238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusama Y., Takamoto M., Kasahara T., Takatsu K., Nariuchi H., Sugane K. Mechanisms of eosinophilia in BALB/c-nu/+ and congenitally athymic BALB/c-nu/nu mice infected with Toxocara canis. Immunology. 1995 Mar;84(3):461–468. [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H., Wang C. R., Yoshimoto T., Sugishita C., Shiroishi T., Matsuzawa A., Nariuchi H. Novel mutant mice secreting soluble CD4 without expression of membrane-bound CD4. Eur J Immunol. 1998 Feb;28(2):403–412. doi: 10.1002/(SICI)1521-4141(199802)28:02<403::AID-IMMU403>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Savigny D. H. In vitro maintenance of Toxocara canis larvae and a simple method for the production of Toxocara ES antigen for use in serodiagnostic tests for visceral larva migrans. J Parasitol. 1975 Aug;61(4):781–782. [PubMed] [Google Scholar]
- Sugane K., Oshima T. Eosinophilia, granuloma formation and migratory behaviour of larvae in the congenitally athymic mouse infected with Toxocara canis. Parasite Immunol. 1982 Sep;4(5):307–318. doi: 10.1111/j.1365-3024.1982.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Swain S. L., Dialynas D. P., Fitch F. W., English M. Monoclonal antibody to L3T4 blocks the function of T cells specific for class 2 major histocompatibility complex antigens. J Immunol. 1984 Mar;132(3):1118–1123. [PubMed] [Google Scholar]
- Takamoto M., Kusama Y., Takatsu K., Nariuchi H., Sugane K. Occurrence of interleukin-5 production by CD4- CD8- (double-negative) T cells in lungs of both normal and congenitally athymic nude mice infected with Toxocara canis. Immunology. 1995 Jun;85(2):285–291. [PMC free article] [PubMed] [Google Scholar]
- Takamoto M., Sugane K. Mechanisms of eosinophilia in Toxocara canis infected mice: in vitro production of interleukin 5 by lung cells of both normal and congenitally athymic nude mice. Parasite Immunol. 1993 Sep;15(9):493–500. doi: 10.1111/j.1365-3024.1993.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Kobayashi A., Miyajima H., Hirano T., Ovary Z. Detection of IgE antibody-forming cells by passive cutaneous anaphylaxis using cell extract from lymphoid organs. J Immunol Methods. 1987 Jan 26;96(1):41–45. doi: 10.1016/0022-1759(87)90365-6. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Ovary Z. Antigen and antibody detection by in vivo methods; a reevaluation of passive cutaneous anaphylactic reactions. J Immunol Methods. 1977;14(3-4):381–390. doi: 10.1016/0022-1759(77)90149-1. [DOI] [PubMed] [Google Scholar]


