Abstract
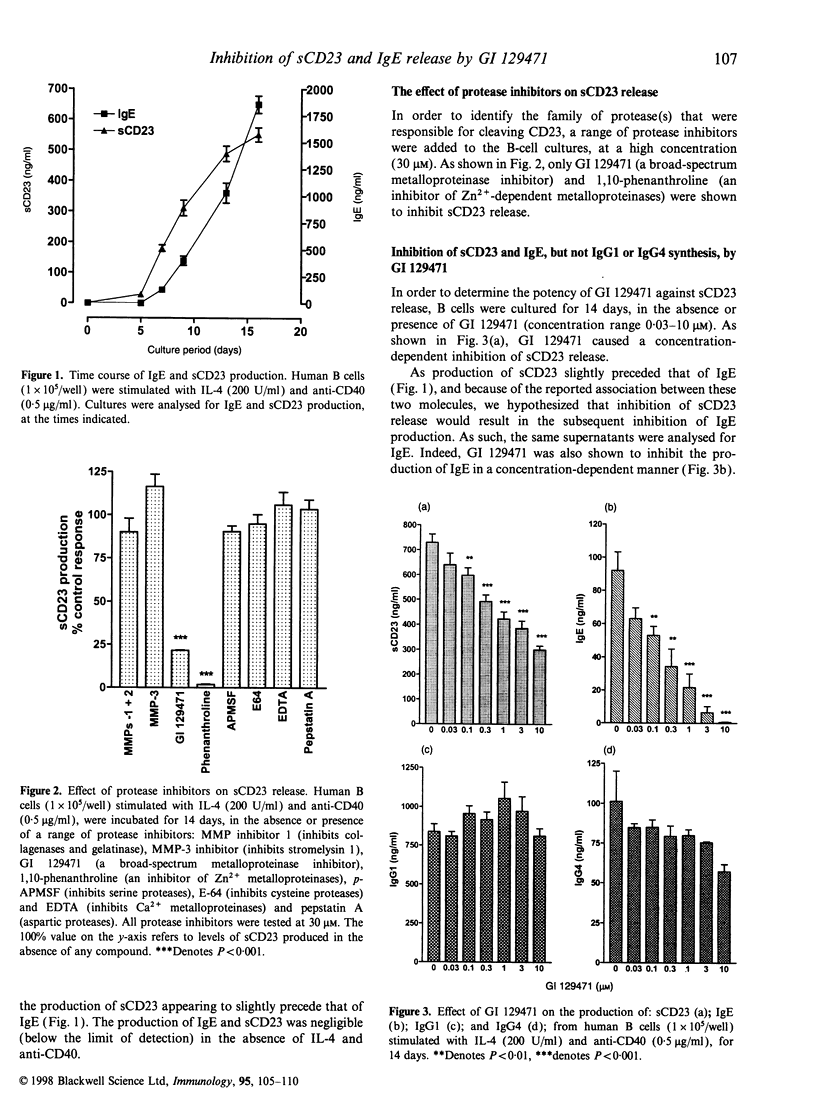
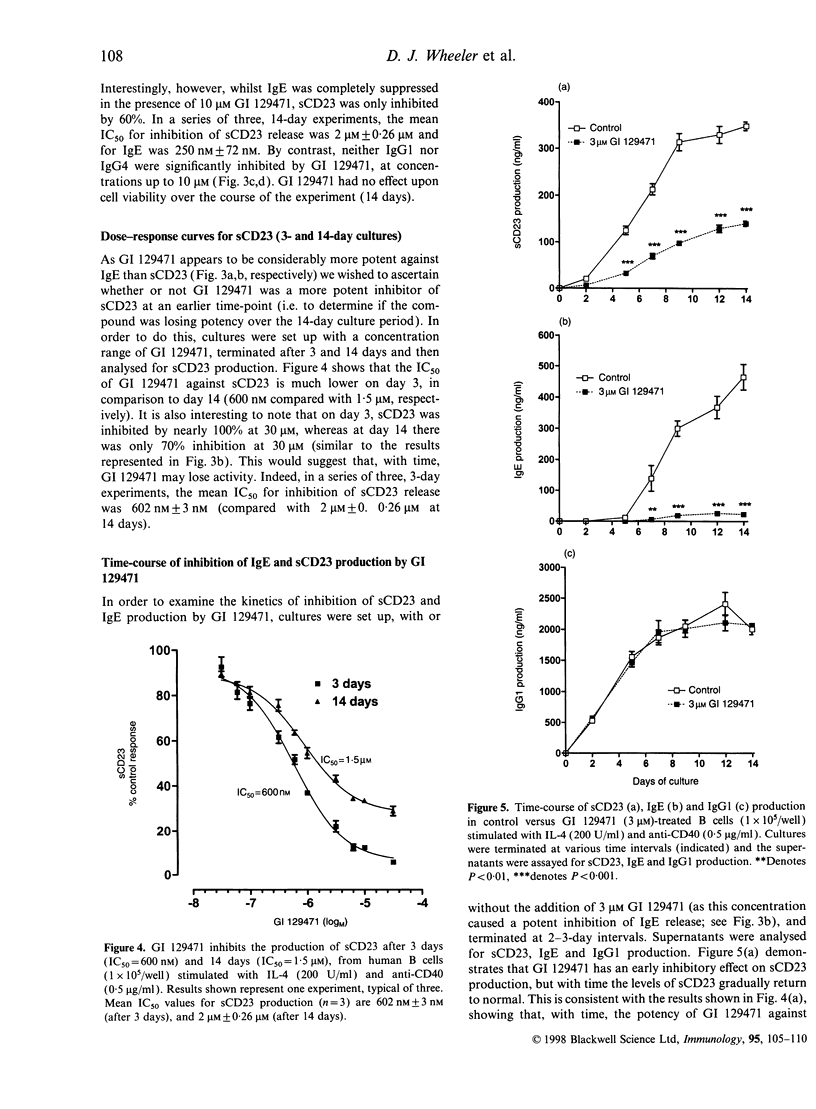
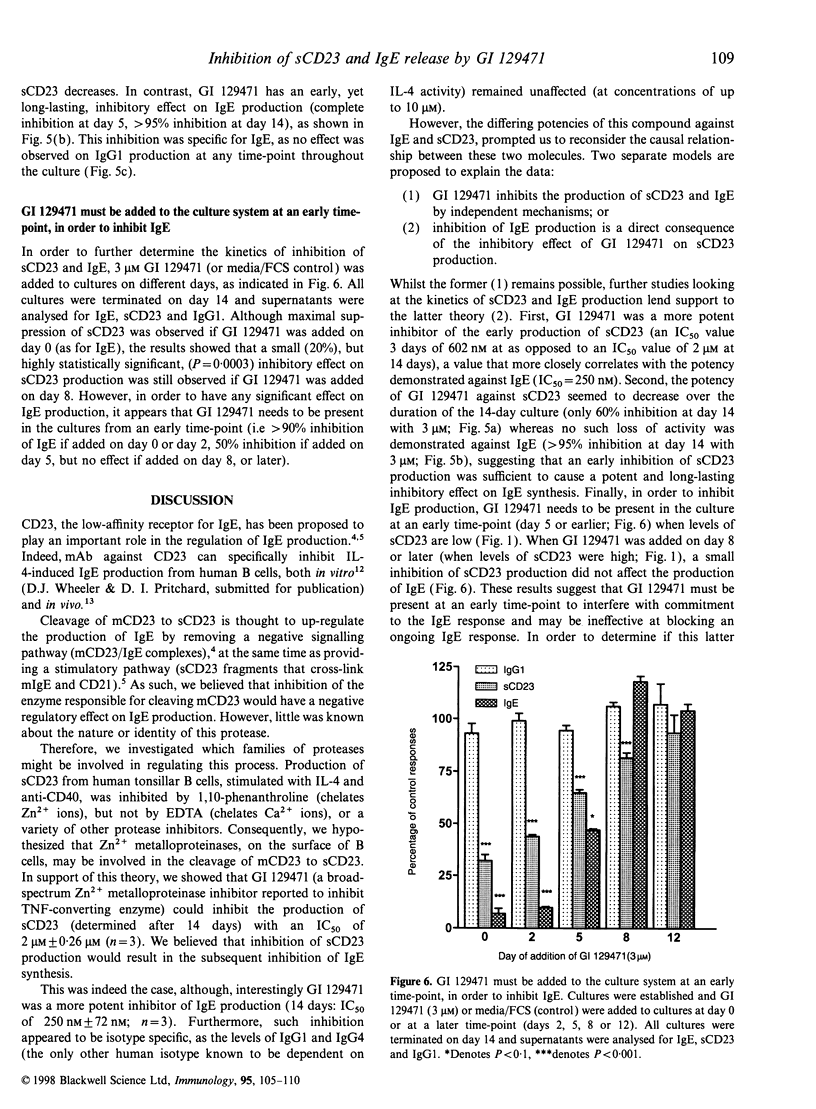
Soluble CD23 (sCD23) has been proposed to play an important role in the up-regulation of immunoglobulin E (IgE) synthesis. Production of sCD23 is dependent on the proteolytic cleavage of membrane CD23, but the protease(s) involved in this process remain unknown. Preliminary data, obtained by testing a panel of protease inhibitors, suggested that this enzyme may be a zinc-dependent metalloproteinase. Therefore, we investigated the effect of a standard hydroxamate-type Zn2+ metalloproteinase inhibitor (GI 129471) on both sCD23 and IgE release from human tonsillar B cells, stimulated with interleukin-4 (IL-4) and anti-CD40. Incubation of cells for 3 days with GI 129471 inhibited the production of sCD23 with an IC50 of 602 nm+/-3 nm (n=3), but by 14 days the activity of the compound against sCD23 had decreased by greater than threefold (IC50 2+/-0.26 microM; n=3). On the other hand, GI 129471 caused a potent inhibition of IgE production, with no apparent loss of activity over the culture period (14 days: IC50 250 nm+/-72 nm; n=3). Time-course studies showed that, despite loss of activity against sCD23, inhibition of sCD23 production early in the culture was able to cause a potent and long-lasting inhibitory effect on IgE. Furthermore, we also showed that the activity of GI 129471 is selective for IgE, as no effect was seen on immunoglobulin G1 (IgG1) or IgG4 production at test concentrations as high as 10 microM. These results support the hypothesis that metalloproteinases may be involved in the proteolytic cleavage of CD23 and subsequent regulation of IgE synthesis. Inhibition of the protease(s) responsible for such cleavage may be of value in the treatment of allergic disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubry J. P., Pochon S., Graber P., Jansen K. U., Bonnefoy J. Y. CD21 is a ligand for CD23 and regulates IgE production. Nature. 1992 Aug 6;358(6386):505–507. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- Bansal A., Roberts T., Hay E. M., Kay R., Pumphrey R. S., Wilson P. B. Soluble CD23 levels are elevated in the serum of patients with primary Sjögren's syndrome and systemic lupus erythematosus. Clin Exp Immunol. 1992 Sep;89(3):452–455. doi: 10.1111/j.1365-2249.1992.tb06979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefoy J. Y., Shields J., Mermod J. J. Inhibition of human interleukin 4-induced IgE synthesis by a subset of anti-CD23/Fc epsilon RII monoclonal antibodies. Eur J Immunol. 1990 Jan;20(1):139–144. doi: 10.1002/eji.1830200120. [DOI] [PubMed] [Google Scholar]
- Christie G., Barton A., Bolognese B., Buckle D. R., Cook R. M., Hansbury M. J., Harper G. P., Marshall L. A., McCord M. E., Moulder K. IgE secretion is attenuated by an inhibitor of proteolytic processing of CD23 (Fc epsilonRII). Eur J Immunol. 1997 Dec;27(12):3228–3235. doi: 10.1002/eji.1830271221. [DOI] [PubMed] [Google Scholar]
- Flores-Romo L., Shields J., Humbert Y., Graber P., Aubry J. P., Gauchat J. F., Ayala G., Allet B., Chavez M., Bazin H. Inhibition of an in vivo antigen-specific IgE response by antibodies to CD23. Science. 1993 Aug 20;261(5124):1038–1041. doi: 10.1126/science.8351517. [DOI] [PubMed] [Google Scholar]
- Ghaderi A. A., Jones V. M., Stanworth D. R. Affinity-purified soluble Fc epsilon RII/CD23 derived from RPMI-8866 cells induces histamine release from human nasal polyp mast cells through a non-IgE-mediated mechanism. Immunol Lett. 1991 Feb;27(2):113–118. doi: 10.1016/0165-2478(91)90137-y. [DOI] [PubMed] [Google Scholar]
- Hellen E. A., Rowlands D. C., Hansel T. T., Kitas G. D., Crocker J. Immunohistochemical demonstration of CD23 expression on lymphocytes in rheumatoid synovitis. J Clin Pathol. 1991 Apr;44(4):293–296. doi: 10.1136/jcp.44.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka A., Sakiyama Y., Nakanishi M., Tomizawa K., Oshika E., Kojima K., Taguchi Y., Kandil E., Matsumoto S. The inductive effect of interleukin-4 on IgG4 and IgE synthesis in human peripheral blood lymphocytes. Clin Exp Immunol. 1990 Mar;79(3):392–396. doi: 10.1111/j.1365-2249.1990.tb08101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserlian D., Lachaux A., Grosjean I., Graber P., Bonnefoy J. Y. Intestinal epithelial cells express the CD23/Fc epsilon RII molecule: enhanced expression in enteropathies. Immunology. 1993 Sep;80(1):90–95. [PMC free article] [PubMed] [Google Scholar]
- Lebman D. A., Coffman R. L. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med. 1988 Sep 1;168(3):853–862. doi: 10.1084/jem.168.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeehan G. M., Becherer J. D., Bast R. C., Jr, Boyer C. M., Champion B., Connolly K. M., Conway J. G., Furdon P., Karp S., Kidao S. Regulation of tumour necrosis factor-alpha processing by a metalloproteinase inhibitor. Nature. 1994 Aug 18;370(6490):558–561. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Ledbetter J. A., Aruffo A. CD40 and its ligand, an essential ligand-receptor pair for thymus-dependent B-cell activation. Immunol Today. 1992 Nov;13(11):431–433. doi: 10.1016/0167-5699(92)90068-I. [DOI] [PubMed] [Google Scholar]
- Plater-Zyberk C., Bonnefoy J. Y. Marked amelioration of established collagen-induced arthritis by treatment with antibodies to CD23 in vivo. Nat Med. 1995 Aug;1(8):781–785. doi: 10.1038/nm0895-781. [DOI] [PubMed] [Google Scholar]
- Saxon A., Feldhaus J., Robins R. A. Single step separation of human T and B cells using AET treated srbc rosettes. J Immunol Methods. 1976;12(3-4):285–288. doi: 10.1016/0022-1759(76)90050-8. [DOI] [PubMed] [Google Scholar]
- Sutton B. J., Gould H. J. The human IgE network. Nature. 1993 Dec 2;366(6454):421–428. doi: 10.1038/366421a0. [DOI] [PubMed] [Google Scholar]
- Wheeler D. J., Robins R. A., Pritchard D. I., Bundick R. V., Shakib F. Peripheral blood based T cell-containing and T cell-depleted culture systems for human IgE synthesis: the role of T cells. Clin Exp Allergy. 1996 Jan;26(1):28–35. doi: 10.1111/j.1365-2222.1996.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Yanagihara Y., Sarfati M., Marsh D., Nutman T., Delespesse G. Serum levels of IgE-binding factor (soluble CD23) in diseases associated with elevated IgE. Clin Exp Allergy. 1990 Jul;20(4):395–401. doi: 10.1111/j.1365-2222.1990.tb02800.x. [DOI] [PubMed] [Google Scholar]
- Yano N., Miyazaki M., Endoh M., Kuramoto T., Eguchi K., Yagame M., Nomoto Y., Sakai H. Increase of CD23-positive cells in peripheral blood from patients with IgA nephropathy and non-IgA proliferative glomerulonephritis. Nephron. 1992;60(4):404–410. doi: 10.1159/000186799. [DOI] [PubMed] [Google Scholar]
- Yu P., Kosco-Vilbois M., Richards M., Köhler G., Lamers M. C. Negative feedback regulation of IgE synthesis by murine CD23. Nature. 1994 Jun 30;369(6483):753–756. doi: 10.1038/369753a0. [DOI] [PubMed] [Google Scholar]


