Abstract
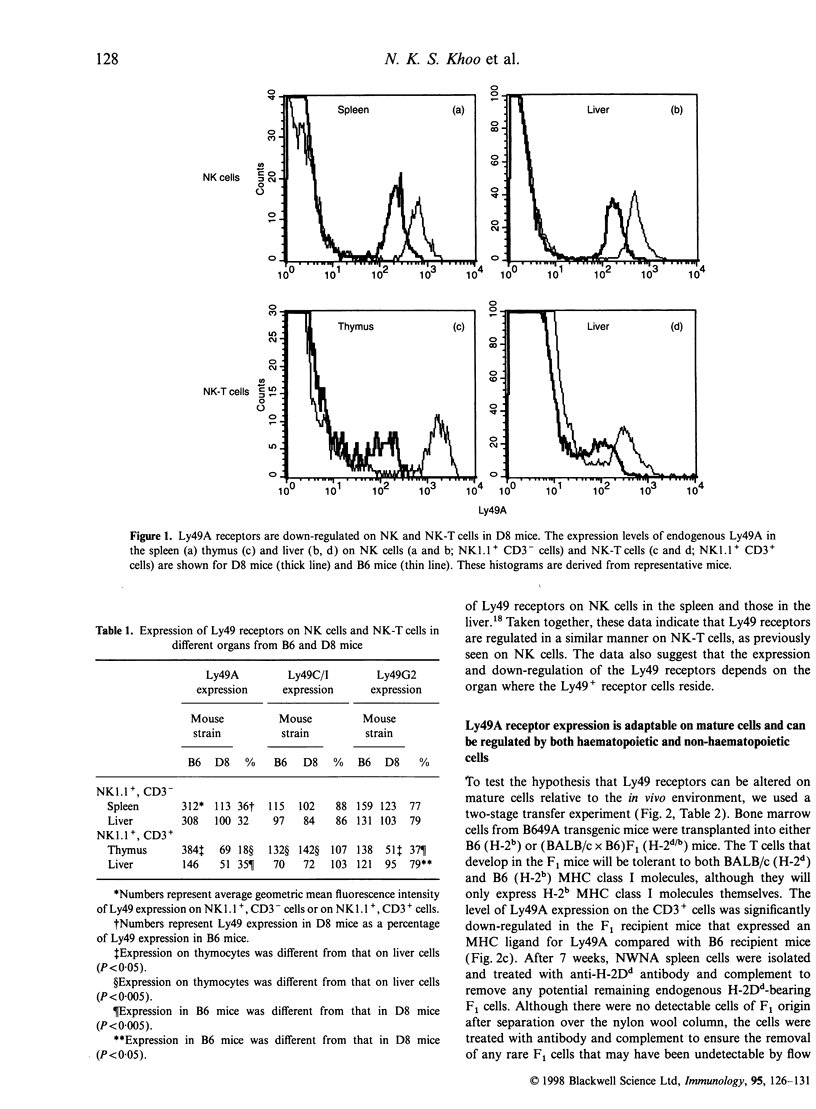
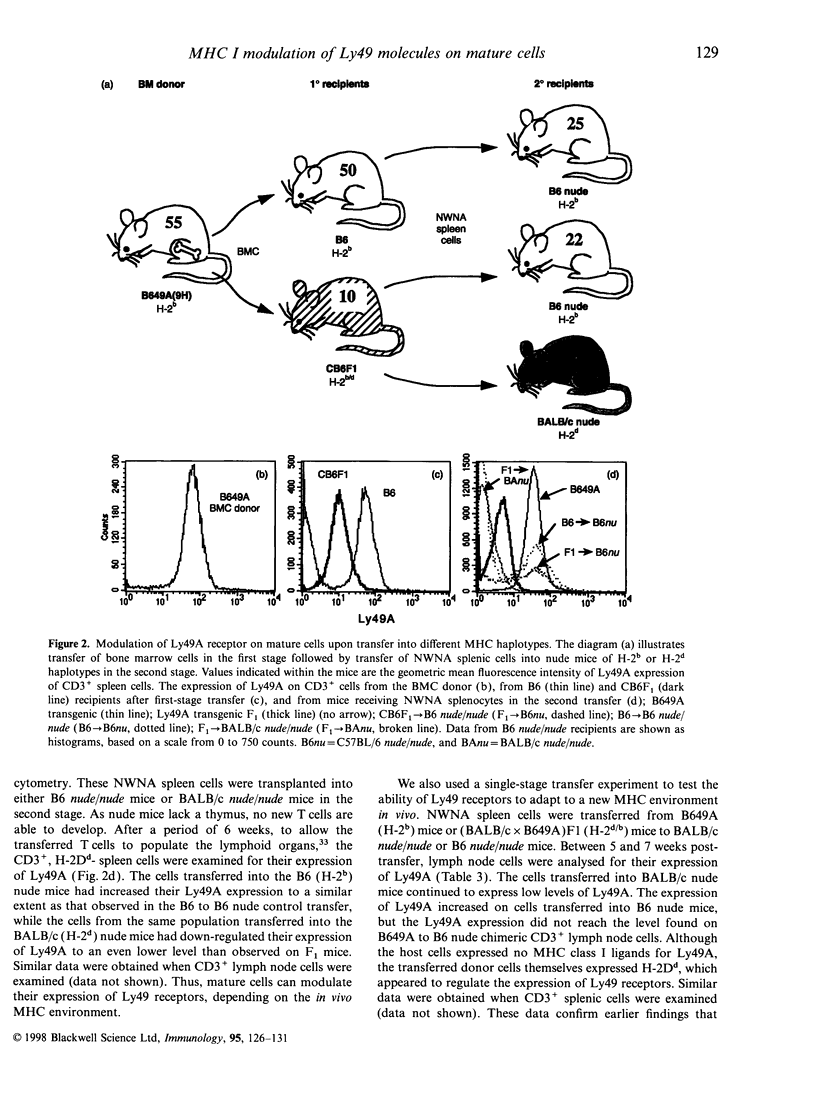
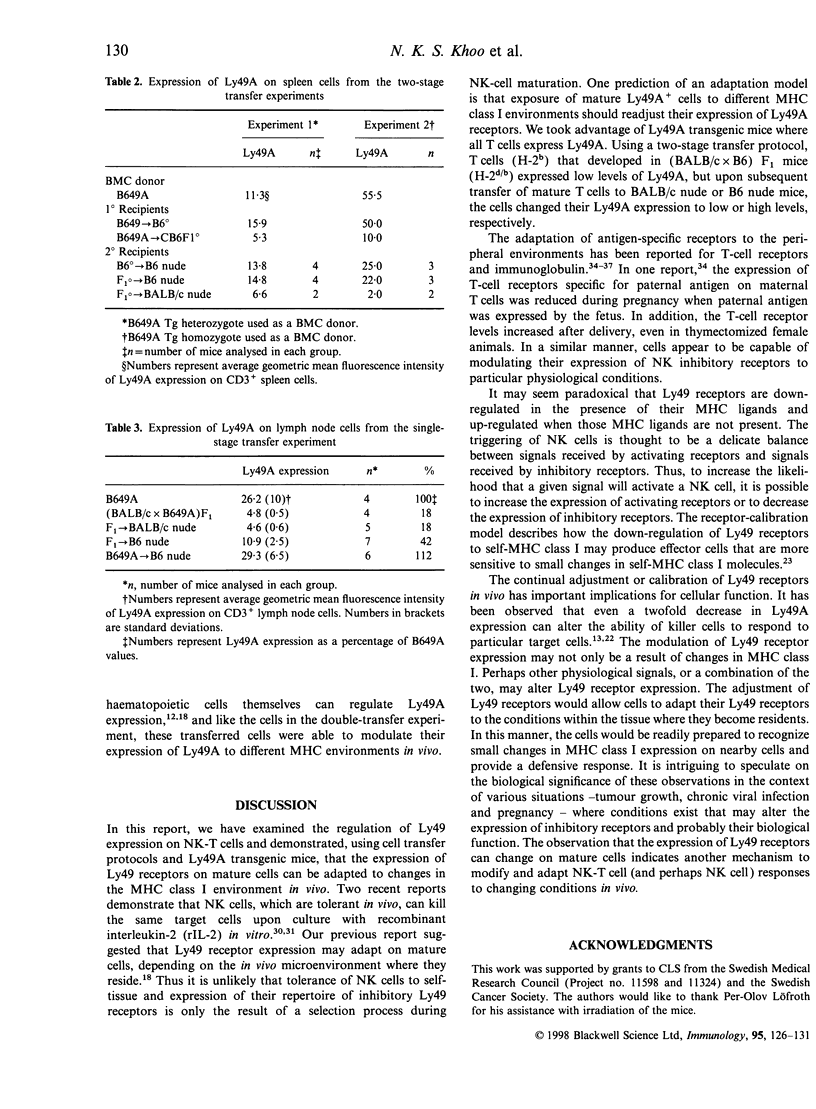
The expression of murine Ly49 receptors on natural killer (NK) cells and NK1.1+ T cells is believed to prevent these cells from responding against normal self-tissues. In this report we investigated whether the expression level of Ly49A was fixed on mature cells or if it could be adapted as the major histocompatibility complex (MHC) class I environment changed in vivo. By transferring peripheral T cells from Ly49A transgenic mice into BALB/c nude/nude and B6 nude/nude mice, we demonstrated that mature cells modulate their Ly49A receptor expression relative to the in vivo MHC class I environment. These results indicated that the expression of the inhibitory Ly49A receptor is not permanently fixed during a maturation and/or education process but rather is adapted to MHC class I changes on the surrounding cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dorfman J. R., Raulet D. H. Major histocompatibility complex genes determine natural killer cell tolerance. Eur J Immunol. 1996 Jan;26(1):151–155. doi: 10.1002/eji.1830260123. [DOI] [PubMed] [Google Scholar]
- Fahlén L., Khoo N. K., Daws M. R., Sentman C. L. Location-specific regulation of transgenic Ly49A receptors by major histocompatibility complex class I molecules. Eur J Immunol. 1997 Aug;27(8):2057–2065. doi: 10.1002/eji.1830270833. [DOI] [PubMed] [Google Scholar]
- Freitas A. A., Agenes F., Coutinho G. C. Cellular competition modulates survival and selection of CD8+ T cells. Eur J Immunol. 1996 Nov;26(11):2640–2649. doi: 10.1002/eji.1830261115. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Jorgensen H., Brink R. A., Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989 Nov 23;342(6248):385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- Gosselin P., Lusignan Y., Brennan J., Takei F., Lemieux S. The NK2.1 receptor is encoded by Ly-49C and its expression is regulated by MHC class I alleles. Int Immunol. 1997 Apr;9(4):533–540. doi: 10.1093/intimm/9.4.533. [DOI] [PubMed] [Google Scholar]
- Held W., Cado D., Raulet D. H. Transgenic expression of the Ly49A natural killer cell receptor confers class I major histocompatibility complex (MHC)-specific inhibition and prevents bone marrow allograft rejection. J Exp Med. 1996 Nov 1;184(5):2037–2041. doi: 10.1084/jem.184.5.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W., Dorfman J. R., Wu M. F., Raulet D. H. Major histocompatibility complex class I-dependent skewing of the natural killer cell Ly49 receptor repertoire. Eur J Immunol. 1996 Oct;26(10):2286–2292. doi: 10.1002/eji.1830261003. [DOI] [PubMed] [Google Scholar]
- Held W., Raulet D. H. Ly49A transgenic mice provide evidence for a major histocompatibility complex-dependent education process in natural killer cell development. J Exp Med. 1997 Jun 16;185(12):2079–2088. doi: 10.1084/jem.185.12.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund P., Ohlén C., Carbone E., Franksson L., Ljunggren H. G., Latour A., Koller B., Kärre K. Recognition of beta 2-microglobulin-negative (beta 2m-) T-cell blasts by natural killer cells from normal but not from beta 2m- mice: nonresponsiveness controlled by beta 2m- bone marrow in chimeric mice. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10332–10336. doi: 10.1073/pnas.88.22.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M. H., Bieberich C., Jay G., Kärre K., Höglund P. Natural killer cell tolerance in mice with mosaic expression of major histocompatibility complex class I transgene. J Exp Med. 1997 Aug 4;186(3):353–364. doi: 10.1084/jem.186.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlhofer F. M., Hunziker R., Reichlin A., Margulies D. H., Yokoyama W. M. Host MHC class I molecules modulate in vivo expression of a NK cell receptor. J Immunol. 1994 Sep 15;153(6):2407–2416. [PubMed] [Google Scholar]
- Karlhofer F. M., Ribaudo R. K., Yokoyama W. M. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992 Jul 2;358(6381):66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- Kung S. K., Miller R. G. Mouse natural killer subsets defined by their target specificity and their ability to be separately rendered unresponsive in vivo. J Immunol. 1997 Mar 15;158(6):2616–2626. [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Liao N. S., Bix M., Zijlstra M., Jaenisch R., Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991 Jul 12;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990 Jul;11(7):237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R. NK1.1+ T cell receptor-alpha/beta+ cells: new clues to their origin, specificity, and function. J Exp Med. 1995 Sep 1;182(3):633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieza M. A., Itoh T., Cui J. Q., Makino Y., Kawano T., Tsuchida K., Koike T., Shirai T., Yagita H., Matsuzawa A. Selective reduction of V alpha 14+ NK T cells associated with disease development in autoimmune-prone mice. J Immunol. 1996 May 15;156(10):4035–4040. [PubMed] [Google Scholar]
- Ohlén C., Kling G., Höglund P., Hansson M., Scangos G., Bieberich C., Jay G., Kärre K. Prevention of allogeneic bone marrow graft rejection by H-2 transgene in donor mice. Science. 1989 Nov 3;246(4930):666–668. doi: 10.1126/science.2814488. [DOI] [PubMed] [Google Scholar]
- Olsson-Alheim M. Y., Salcedo M., Ljunggren H. G., Kärre K., Sentman C. L. NK cell receptor calibration: effects of MHC class I induction on killing by Ly49Ahigh and Ly49Alow NK cells. J Immunol. 1997 Oct 1;159(7):3189–3194. [PubMed] [Google Scholar]
- Olsson M. Y., Kärre K., Sentman C. L. Altered phenotype and function of natural killer cells expressing the major histocompatibility complex receptor Ly-49 in mice transgenic for its ligand. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1649–1653. doi: 10.1073/pnas.92.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Gumperz J. E., Parham P., Lanier L. L. Superantigen-dependent, cell-mediated cytotoxicity inhibited by MHC class I receptors on T lymphocytes. Science. 1995 Apr 21;268(5209):403–405. doi: 10.1126/science.7716542. [DOI] [PubMed] [Google Scholar]
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991 Mar 8;251(4998):1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Salcedo M., Diehl A. D., Olsson-Alheim M. Y., Sundbäck J., Van Kaer L., Kärre K., Ljunggren H. G. Altered expression of Ly49 inhibitory receptors on natural killer cells from MHC class I-deficient mice. J Immunol. 1997 Apr 1;158(7):3174–3180. [PubMed] [Google Scholar]
- Schönrich G., Kalinke U., Momburg F., Malissen M., Schmitt-Verhulst A. M., Malissen B., Hämmerling G. J., Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991 Apr 19;65(2):293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- Sentman C. L., Hackett J., Jr, Kumar V., Bennett M. Identification of a subset of murine natural killer cells that mediates rejection of Hh-1d but not Hh-1b bone marrow grafts. J Exp Med. 1989 Jul 1;170(1):191–202. doi: 10.1084/jem.170.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentman C. L., Olsson M. Y., Kärre K. Missing self recognition by natural killer cells in MHC class I transgenic mice. A 'receptor calibration' model for how effector cells adapt to self. Semin Immunol. 1995 Apr;7(2):109–119. doi: 10.1006/smim.1995.0015. [DOI] [PubMed] [Google Scholar]
- Sumida T., Sakamoto A., Murata H., Makino Y., Takahashi H., Yoshida S., Nishioka K., Iwamoto I., Taniguchi M. Selective reduction of T cells bearing invariant V alpha 24J alpha Q antigen receptor in patients with systemic sclerosis. J Exp Med. 1995 Oct 1;182(4):1163–1168. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundbäck J., Kärre K., Sentman C. L. Cloning of minimally divergent allelic forms of the natural killer (NK) receptor Ly-49C, differentially controlled by host genes in the MHC and NK gene complexes. J Immunol. 1996 Nov 1;157(9):3936–3942. [PubMed] [Google Scholar]
- Sykes M., Harty M. W., Karlhofer F. M., Pearson D. A., Szot G., Yokoyama W. Hematopoietic cells and radioresistant host elements influence natural killer cell differentiation. J Exp Med. 1993 Jul 1;178(1):223–229. doi: 10.1084/jem.178.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafuri A., Alferink J., Möller P., Hämmerling G. J., Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995 Oct 27;270(5236):630–633. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- Takei F., Brennan J., Mager D. L. The Ly-49 family: genes, proteins and recognition of class I MHC. Immunol Rev. 1997 Feb;155:67–77. doi: 10.1111/j.1600-065x.1997.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari A. P., Zlotnik A. Mouse NK1.1+ T cells: a new family of T cells. Immunol Today. 1996 Feb;17(2):71–76. doi: 10.1016/0167-5699(96)80582-2. [DOI] [PubMed] [Google Scholar]
- Zimmer J., Donato L., Hanau D., Cazenave J. P., Tongio M. M., Moretta A., de la Salle H. Activity and phenotype of natural killer cells in peptide transporter (TAP)-deficient patients (type I bare lymphocyte syndrome). J Exp Med. 1998 Jan 5;187(1):117–122. doi: 10.1084/jem.187.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]