Abstract
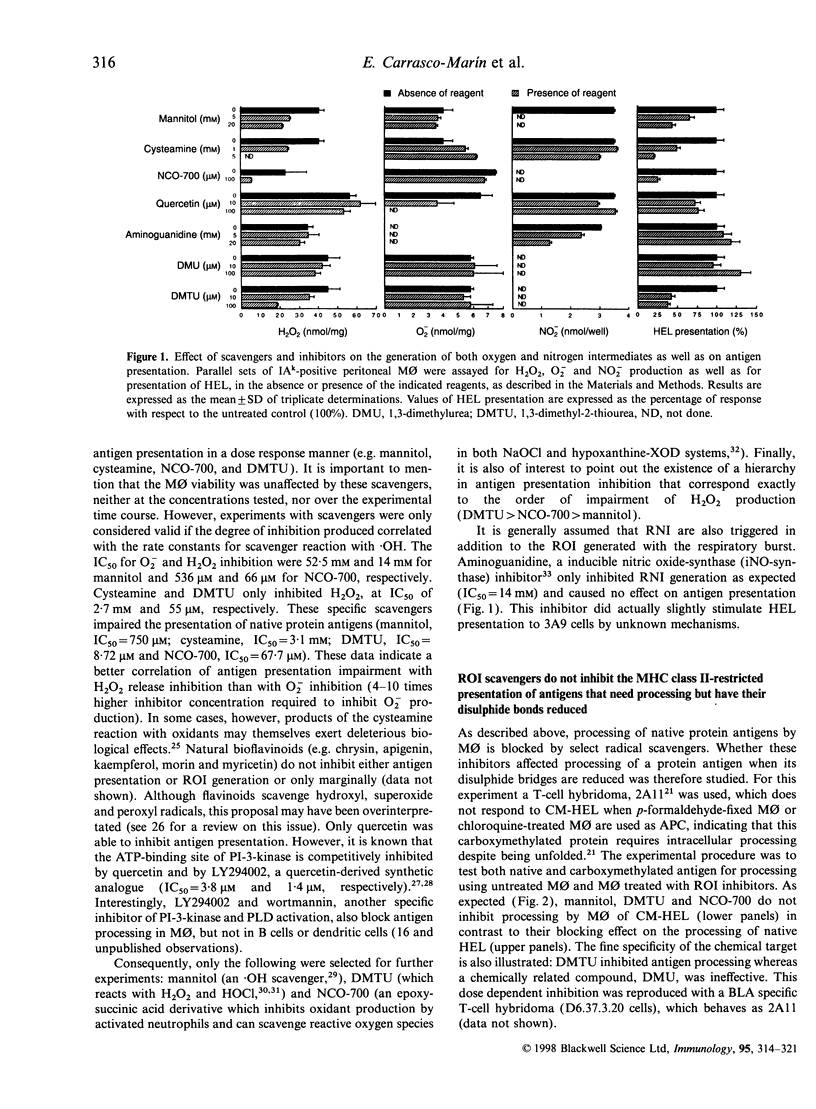
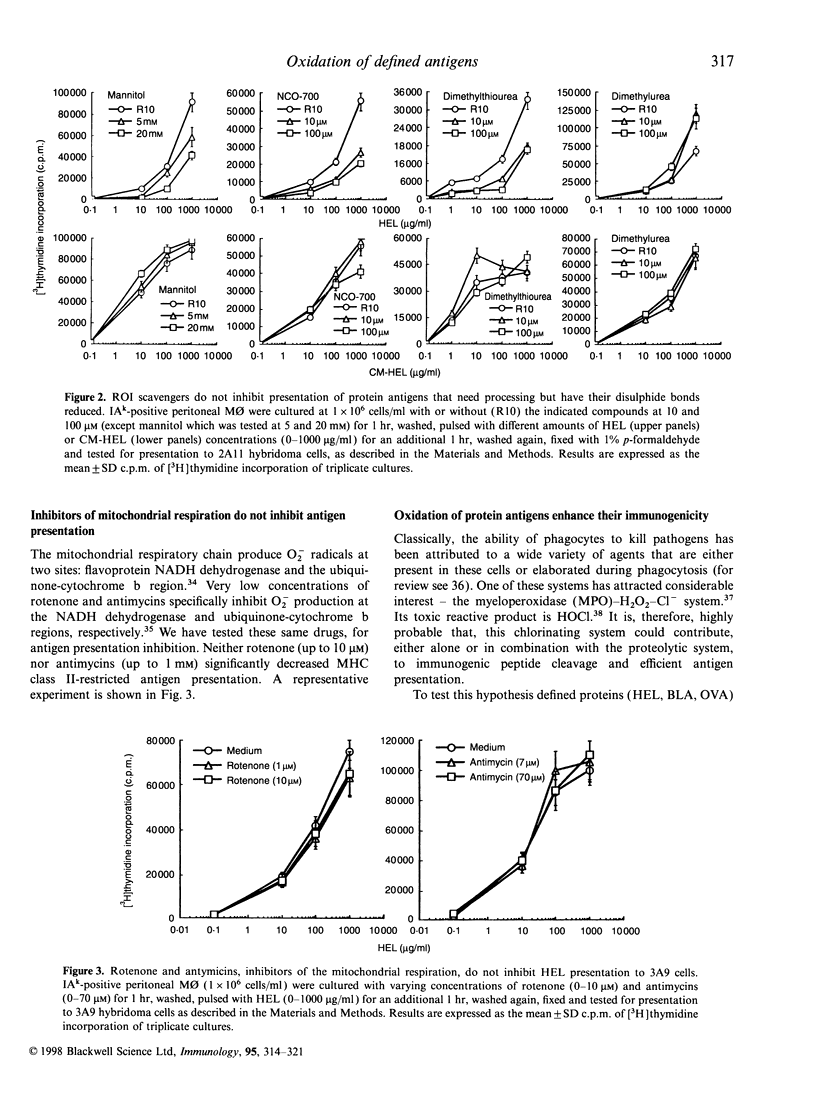
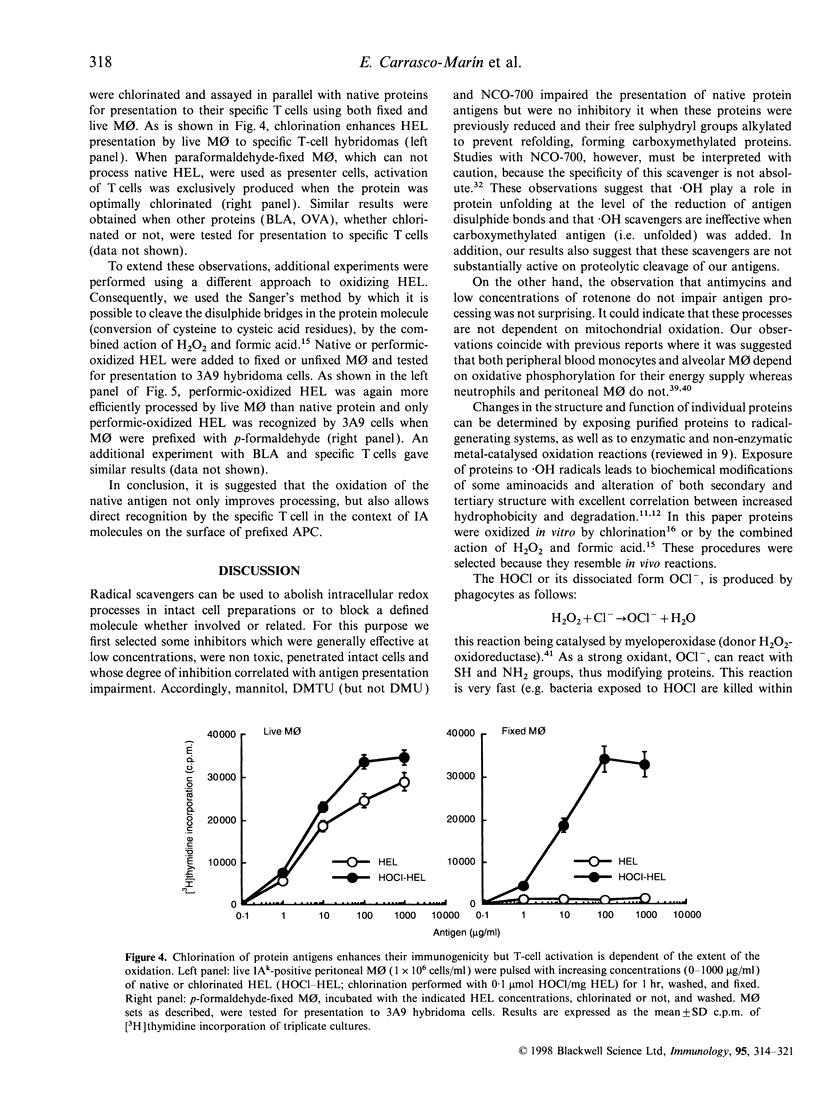
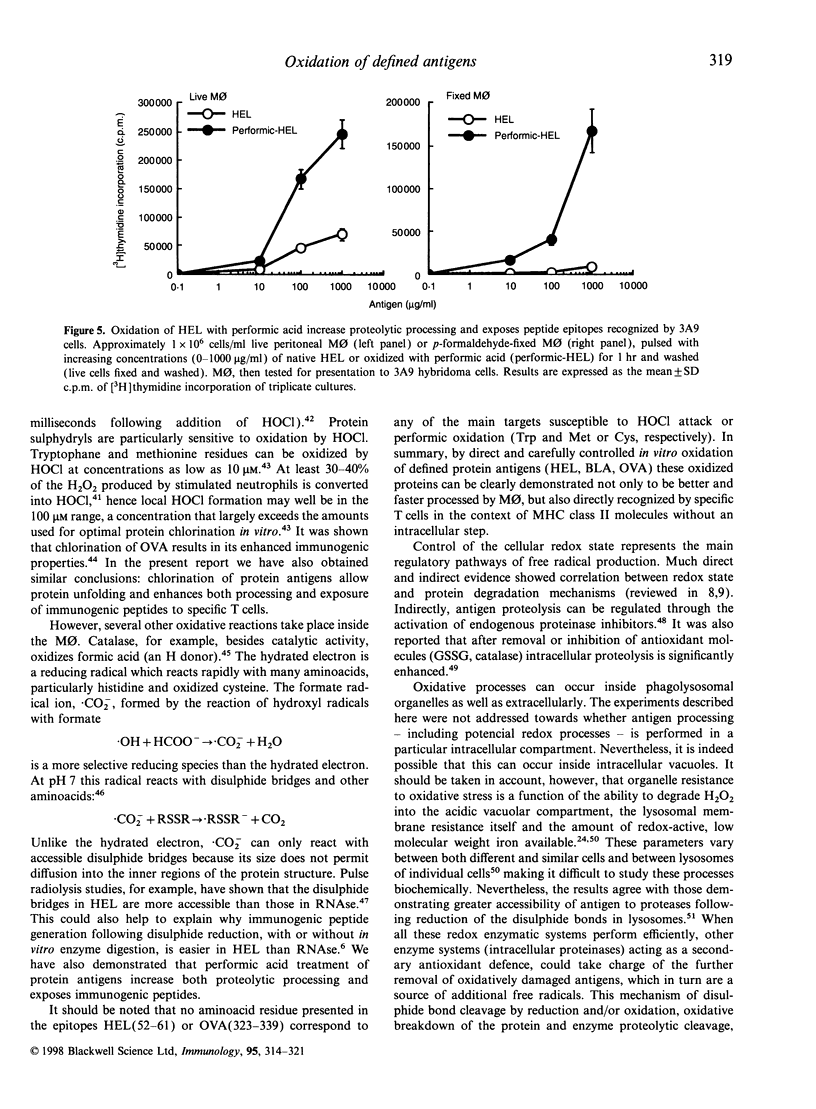
The participation of oxidative mechanisms in major histocompatibility complex (MHC) class II-restricted antigen presentation was studied in vitro. In general, antigen processing is inhibited when peritoneal macrophages (MO) are incubated with scavengers of reactive oxygen intermediates (ROI): mannitol (an.OH scavenger), dimethylurea (DMTU, which reacts with H2O2 and HOCl) and NCO-700 (an epoxysuccinic acid derivative which inhibits oxidant production by activated phagocytes and can scavenge reactive oxygen species in both NaOCl and hypoxanthine (XOD) systems). However, neither rotenone and antimycins (inhibitors of O-2 production at the NADH dehydrogenase and ubiquinone-cytochrome b regions, respectively) nor aminoguanidine (an inducible nitric oxide synthase inhibitor) impaired antigen presentation, thus indirectly discarding the participation of mitochondrial oxidation and reactive nitrogen intermediates (RNI) in antigen processing. ROI scavengers do not inhibit the MHC class II-restricted presentation of antigens that need processing but have their disulphide bonds reduced. It can be shown that oxidation of protein antigens (either by chlorination or performic acid treatment) allow protein unfolding and enhance both processing and exposure of immunogenic epitopes to specific T cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E., Bisby R. H., Cundall R. B., Redpath J. L., Willson R. L. Selective free radical reactions with proteins and enzymes: the inactivation of ribonuclease. Radiat Res. 1972 Feb;49(2):290–299. [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Albrich J. M., McCarthy C. A., Hurst J. K. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci U S A. 1981 Jan;78(1):210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P. M., Strydom D. J., Unanue E. R. Processing of lysozyme by macrophages: identification of the determinant recognized by two T-cell hybridomas. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2489–2493. doi: 10.1073/pnas.81.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Hoey B. M., Butler J. The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem J. 1988 Nov 15;256(1):251–255. doi: 10.1042/bj2560251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Production of superoxide by phagocytic leukocytes: a paradigm for stimulus-response phenomena. Curr Top Cell Regul. 1986;28:183–208. doi: 10.1016/b978-0-12-152828-7.50006-8. [DOI] [PubMed] [Google Scholar]
- Bors W., Heller W., Michel C., Saran M. Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods Enzymol. 1990;186:343–355. doi: 10.1016/0076-6879(90)86128-i. [DOI] [PubMed] [Google Scholar]
- Carrasco-Marín E., Alvarez-Domínguez C., Leyva-Cobián F. Wortmannin, an inhibitor of phospholipase D activation, selectively blocks major histocompatibility complex class II-restricted antigen presentation. Eur J Immunol. 1994 Sep;24(9):2031–2039. doi: 10.1002/eji.1830240915. [DOI] [PubMed] [Google Scholar]
- Carrasco-Marín E., Alvarez-Domínguez C., López-Mato P., Martínez-Palencia R., Leyva-Cobián F. Iron salts and iron-containing porphyrins block presentation of protein antigens by macrophages to MHC class II-restricted T cells. Cell Immunol. 1996 Aug 1;171(2):173–185. doi: 10.1006/cimm.1996.0192. [DOI] [PubMed] [Google Scholar]
- Collins D. S., Unanue E. R., Harding C. V. Reduction of disulfide bonds within lysosomes is a key step in antigen processing. J Immunol. 1991 Dec 15;147(12):4054–4059. [PubMed] [Google Scholar]
- Curtis W. E., Muldrow M. E., Parker N. B., Barkley R., Linas S. L., Repine J. E. N,N'-dimethylthiourea dioxide formation from N,N'-dimethylthiourea reflects hydrogen peroxide concentrations in simple biological systems. Proc Natl Acad Sci U S A. 1988 May;85(10):3422–3425. doi: 10.1073/pnas.85.10.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. J., Goldberg A. L. Proteins damaged by oxygen radicals are rapidly degraded in extracts of red blood cells. J Biol Chem. 1987 Jun 15;262(17):8227–8234. [PubMed] [Google Scholar]
- Del Pozo V., De Andrés B., Martín E., Cárdaba B., Fernández J. C., Gallardo S., Tramón P., Leyva-Cobian F., Palomino P., Lahoz C. Eosinophil as antigen-presenting cell: activation of T cell clones and T cell hybridoma by eosinophils after antigen processing. Eur J Immunol. 1992 Jul;22(7):1919–1925. doi: 10.1002/eji.1830220736. [DOI] [PubMed] [Google Scholar]
- Diment S. Different roles for thiol and aspartyl proteases in antigen presentation of ovalbumin. J Immunol. 1990 Jul 15;145(2):417–422. [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Foote C. S., Goyne T. E., Lehrer R. I. Assessment of chlorination by human neutrophils. Nature. 1983 Feb 24;301(5902):715–716. doi: 10.1038/301715a0. [DOI] [PubMed] [Google Scholar]
- Fukami J. I., Yamamoto I., Casida J. E. Metabolism of rotenone in vitro by tissue homogenates from mammals and insects. Science. 1967 Feb 10;155(3763):713–716. doi: 10.1126/science.155.3763.713. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D., Witz G., Amoruso M., Troll W. Protease inhibitors antagonize the activation of polymorphonuclear leukocyte oxygen consumption. Biochem Biophys Res Commun. 1979 Jun 13;88(3):854–860. doi: 10.1016/0006-291x(79)91487-6. [DOI] [PubMed] [Google Scholar]
- Hazen S. L., d'Avignon A., Anderson M. M., Hsu F. F., Heinecke J. W. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to oxidize alpha-amino acids to a family of reactive aldehydes. Mechanistic studies identifying labile intermediates along the reaction pathway. J Biol Chem. 1998 Feb 27;273(9):4997–5005. doi: 10.1074/jbc.273.9.4997. [DOI] [PubMed] [Google Scholar]
- Jensen P. E. Acidification and disulfide reduction can be sufficient to allow intact proteins to bind class II MHC. J Immunol. 1993 Apr 15;150(8 Pt 1):3347–3356. [PubMed] [Google Scholar]
- Jensen P. E. Reduction of disulfide bonds during antigen processing: evidence from a thiol-dependent insulin determinant. J Exp Med. 1991 Nov 1;174(5):1121–1130. doi: 10.1084/jem.174.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Takaku F., Sakamoto S. Possible involvement of proteases in superoxide production by human polymorphonuclear leukocytes. FEBS Lett. 1979 Mar 15;99(2):275–278. doi: 10.1016/0014-5793(79)80971-0. [DOI] [PubMed] [Google Scholar]
- Lepay D. A., Nathan C. F., Steinman R. M., Murray H. W., Cohn Z. A. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J Exp Med. 1985 May 1;161(5):1079–1096. doi: 10.1084/jem.161.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. L., Oliver C. N., Fulks R. M., Stadtman E. R. Turnover of bacterial glutamine synthetase: oxidative inactivation precedes proteolysis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J. B. Disulphide reduction in lysosomes. The role of cysteine. Biochem J. 1986 Jul 1;237(1):271–272. doi: 10.1042/bj2370271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz J., Chain B. M., Olszowska E., Olszowski S., Zgliczyński J. M. Enhancement of immunogenic properties of ovalbumin as a result of its chlorination. Int J Biochem. 1991;23(12):1393–1395. doi: 10.1016/0020-711x(91)90280-z. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz J. Neutrophil chloramines: missing links between innate and acquired immunity. Immunol Today. 1997 Dec;18(12):577–580. doi: 10.1016/s0167-5699(97)01161-4. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz J., Olszowska E., Olszowski S., Zgliczynski J. M. Enhancement of trinitrophenyl-specific humoral response to TNP proteins as the result of carrier chlorination. Immunology. 1992 Jul;76(3):385–388. [PMC free article] [PubMed] [Google Scholar]
- Matter W. F., Brown R. F., Vlahos C. J. The inhibition of phosphatidylinositol 3-kinase by quercetin and analogs. Biochem Biophys Res Commun. 1992 Jul 31;186(2):624–631. doi: 10.1016/0006-291x(92)90792-j. [DOI] [PubMed] [Google Scholar]
- Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R., Currie M. G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- Nilsson E., Ghassemifar R., Brunk U. T. Lysosomal heterogeneity between and within cells with respect to resistance against oxidative stress. Histochem J. 1997 Nov-Dec;29(11-12):857–865. doi: 10.1023/a:1026441907803. [DOI] [PubMed] [Google Scholar]
- Olszowska E., Olszowski S., Zgliczyński J. M., Stelmaszyńska T. Enhancement of proteinase-mediated degradation of proteins modified by chlorination. Int J Biochem. 1989;21(7):799–805. doi: 10.1016/0020-711x(89)90213-9. [DOI] [PubMed] [Google Scholar]
- Pacifici R. E., Davies K. J. Protein degradation as an index of oxidative stress. Methods Enzymol. 1990;186:485–502. doi: 10.1016/0076-6879(90)86143-j. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Pisoni R. L., Acker T. L., Lisowski K. M., Lemons R. M., Thoene J. G. A cysteine-specific lysosomal transport system provides a major route for the delivery of thiol to human fibroblast lysosomes: possible role in supporting lysosomal proteolysis. J Cell Biol. 1990 Feb;110(2):327–335. doi: 10.1083/jcb.110.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L. Destruction of proteins as a creative force. Immunol Today. 1997 Jun;18(6):269–271. doi: 10.1016/s0167-5699(97)80021-7. [DOI] [PubMed] [Google Scholar]
- Redpath J. L. The use of selective free radical probes to study active sites in enzymes and viruses. Methods Enzymol. 1984;105:491–501. doi: 10.1016/s0076-6879(84)05068-0. [DOI] [PubMed] [Google Scholar]
- Reiss M., Roos D. Differences in oxygen metabolism of phagocytosing monocytes and neutrophils. J Clin Invest. 1978 Feb;61(2):480–488. doi: 10.1172/JCI108959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J Exp Med. 1979 Jan 1;149(1):27–39. doi: 10.1084/jem.149.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein D., Roska A. K., Lipsky P. E. Antigen presentation by liver sinusoidal lining cells after antigen exposure in vivo. J Immunol. 1987 Mar 1;138(5):1377–1382. [PubMed] [Google Scholar]
- Sanger F. Fractionation of oxidized insulin. Biochem J. 1949;44(1):126–128. doi: 10.1042/bj0440126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstätter I. U., Browne K., Harris A., Hyslop P. A., Jackson J. H., Quehenberger O., Cochrane C. G. Mechanisms of hypochlorite injury of target cells. J Clin Invest. 1990 Feb;85(2):554–562. doi: 10.1172/JCI114472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj R. J., Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Oxidative peptide cleavage and decarboxylation by the MPO-H2O2-Cl- antimicrobial system. Infect Immun. 1974 Feb;9(2):255–260. doi: 10.1128/iai.9.2.255-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R. Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med. 1990;9(4):315–325. doi: 10.1016/0891-5849(90)90006-5. [DOI] [PubMed] [Google Scholar]
- Stubbs M., Kühner A. V., Glass E. A., David J. R., Karnovsky M. L. Metabolic and functonal studies on activated mouse macrophages. J Exp Med. 1973 Feb 1;137(2):537–542. doi: 10.1084/jem.137.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawara K., Fujisawa S., Nakai K. Effect of NCO-700, an inhibitor of thiol protease, on reactive oxygen production by chemotactic peptide-stimulated rabbit peripheral granulocytes. Experientia. 1988 Apr 15;44(4):346–347. doi: 10.1007/BF01961277. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980 Nov 1;191(2):421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadangos J. A., Riese R. J., Peters C., Chapman H. A., Ploegh H. L. Degradation of mouse invariant chain: roles of cathepsins S and D and the influence of major histocompatibility complex polymorphism. J Exp Med. 1997 Aug 18;186(4):549–560. doi: 10.1084/jem.186.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince G. S., Dean R. T. Is enhanced free radical flux associated with increased intracellular proteolysis? FEBS Lett. 1987 Jun 1;216(2):253–256. doi: 10.1016/0014-5793(87)80700-7. [DOI] [PubMed] [Google Scholar]
- Vlahos C. J., Matter W. F., Hui K. Y., Brown R. F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem. 1994 Feb 18;269(7):5241–5248. [PubMed] [Google Scholar]
- Wakamatsu N., Kominami E., Takio K., Katunuma N. Three forms of thiol proteinase inhibitor from rat liver formed depending on the oxidation-reduction state of a sulfhydryl group. J Biol Chem. 1984 Nov 25;259(22):13832–13838. [PubMed] [Google Scholar]
- Wasil M., Halliwell B., Grootveld M., Moorhouse C. P., Hutchison D. C., Baum H. The specificity of thiourea, dimethylthiourea and dimethyl sulphoxide as scavengers of hydroxyl radicals. Their protection of alpha 1-antiproteinase against inactivation by hypochlorous acid. Biochem J. 1987 May 1;243(3):867–870. doi: 10.1042/bj2430867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Regiani S. Neutrophils degrade subendothelial matrices in the presence of alpha-1-proteinase inhibitor. Cooperative use of lysosomal proteinases and oxygen metabolites. J Clin Invest. 1984 May;73(5):1297–1303. doi: 10.1172/JCI111332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Hosaka Y. Cells process exogenous proteins for recognition by cytotoxic T lymphocytes. Science. 1988 Feb 5;239(4840):637–640. doi: 10.1126/science.3257585. [DOI] [PubMed] [Google Scholar]


